Types of cast iron
Carbon is present in such an alloy in the form:
- cementite;
- graphite;
- graphite + cementite.
Castings containing carbon in the form of cementite have a characteristic light sheen and are called white.
Dark graphite in combination with a metal alloy gives the castings a gray color. The configuration of graphite inclusions affects the properties of forgings. Based on these properties, cast iron is divided into:
- grey;
- malleable;
- high strength;
- special purpose.
The photo shows different types of graphite inclusions. They can be lamellar, spherical or flake-shaped.
Malleable cast iron is characterized by graphite inclusions in the form of flakes.
Types of alloys: white and gray
Diagram of the microstructure of ductile cast iron.
The structure of white cast iron is formed due to rapid cooling during solidification. With this technology, carbon dissolved at high temperatures does not have time to separate into a separate structural component and remains in a bound form (cementite or iron carbide Fe3C). Its presence determines the properties of hardness, wear resistance and brittleness.
Since the cooling rate plays a decisive role in the formation of the structure, the thickness of the castings is important. If the cross-section is too large (more than 50 - 60 mm), it is difficult to adjust the required cooling rate and obtain the required graphite-free structure throughout the entire thickness.
White alloys are often called pig alloys, since they are not used in themselves, but serve as an intermediate alloy, which is either annealed into malleable iron (DC) or melted into steel.
The technology for producing gray cast iron involves slow cooling during solidification plus additional modification with silicon in the amount of 1-3% (silicon enhances graphitization), which allows dissolved graphite to separate out in the form of separate inclusions.
The structure of cast iron with the resulting graphite inclusions forms a lower hardness of the material (than with cementite) and allows it to be processed by cutting. The shape and dispersion of graphite, the structure of the metal base determine the properties and types of cast iron alloy material: gray (SG), high-strength (HF).
Features of the production of malleable cast iron
Carbon in this type of cast iron is present in the range from 2.4 to 2.8%.
It also includes Si, Mn, S, P, the amount of which depends on the required properties of the material. Malleable cast iron is produced from castings of the white variety. In them, carbon is completely bound with iron and is represented by iron carbide (cementite Fe3C). When annealing workpieces at a temperature of 950-970°C, graphite is liberated from iron carbide and austenite (A). As a result, it crystallizes, forming the appearance of flakes. The final formation of graphite flakes in cast iron occurs in the temperature range of 760–720°C, which is demonstrated in the Fe–Fe3C diagram.
On it: A is austenite, representing solid inclusions of carbon atoms in the structure of the iron cell; G is graphite; C is cementite; P – pearlite, which is a compound of ferrite and cementite in the eutectoid region during the decomposition of austenite.
The thermal annealing process is carried out in two stages:
- First, the workpieces are heated to 950–1000°C and kept heated until ledeburite (cementite + austenite) decomposes into graphite and austenite.
- Then the workpieces are gradually cooled to a temperature range of 760–720°C, where austenite gives additional cementite (secondary), which is part of the pearlite. With further cooling, pearlite decomposes into ferrite and graphite.
Types of malleable cast iron
The structural composition of cast iron castings depends on the conditions of the annealing technology. It happens:
- ferritic;
- pearlite;
- ferritic-pearlitic.
The ferritic type of products contains ferrite and flake graphite. The pearlitic type consists of pearlite and flake graphite. Ferrite-pearlite contains ferrite, pearlite and graphite flakes.
The structure of each type is shown in the diagrams:
Perlite-based cast iron can be obtained by cooling the casting faster in the decomposition zone. Then, along with ferrite, there will be pearlite in the structure. It will persist with further, rather slow cooling of the alloy below 727°C.
Important! The structure of malleable cast iron depends on the processing temperature and the alloying elements included in its composition.
In practice, the first two types of cast blanks are mainly used (photos and diagrams are given below).
Ferritic type of castings (photo and diagram)
Pearlite type of castings (photo and diagram)
Which iron-carbon alloy is called cast iron?
Cast irons are alloys of iron and carbon containing more than 2.14% carbon.
Interesting materials:
How to evaluate the quality of an employee's work? How to evaluate an employee's performance? How to answer questions when applying for a job? How to respond to gratitude for work? How to respond to a job advertisement? How to respond to a job refusal? How to respond to a job offer with consent? How to transfer the CEO to remote work? How to transfer an employee to a rotational work schedule? How to transfer an employee from his main place of work to an external part-time job?
Properties of malleable cast irons
The technical characteristics and properties of malleable cast iron are determined by the carbon content in the form of graphite, as well as silicon.
For the pearlite type - also chromium and manganese. The structural difference is also reflected in the properties of the products. For example, the ferritic type of castings has less hardness than pearlitic ones, but it is characterized by greater ductility.
Flaky graphite inclusions give products high strength with fairly good ductility. They are capable of undergoing plastic deformation at indoor temperatures. This is where their name “malleable” comes from. It is conditional and does not mean that products from such cast iron can be produced by forging. For their manufacture, the method of casting parts is used.
One of the significant advantages of malleable workpieces is the constancy of their properties over the entire cross section, as well as the absence of internal stresses.
The physical and mechanical characteristics of such castings are between those of gray cast iron and steel. They have:
- good fluidity in liquid form;
- property of vibration absorption under periodically repeated loads;
- good wear resistance;
- resistant to corrosion, so they are not affected by moisture, chemical reagents, including flue gas.
- high density, for example, a workpiece having a thickness of 7-8 mm can withstand pressure during hydraulic tests within 40 atmospheres.
This makes it possible to use castings for the production of various products in the gas and water supply sector.
At low temperatures, under the influence of dynamic loads, the material can become brittle.
Menu
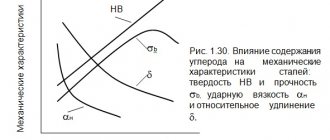
The microstructure of cast iron (Table 1) depends on the cooling rate of the metal: with rapid cooling there will be white cast iron (carbon is in a chemically bound state in the form of cementite and ledeburite), and with slow cooling there will be gray cast iron (carbon is in the form of graphite).Table 1. Grades and mechanical properties of various types of cast iron.
| σВ, MPa | NV | δ | ||
| gray | SCH10 | 100 | 120…150 | |
| SCH15 | 150 | 130…241 | ||
| … | … | … | ||
| SCH35 | 350 | 179…290 | ||
| High strength | HF35 | 350 | 140…170 | 22 |
| HF40 | 400 | 140…202 | 15 | |
| … | … | … | … | |
| HF100 | 1000 | 270…360 | 2 | |
| Malleable | KCh30-6 | 300 | 163 | 6 |
| KCH33-8 | 330 | 163 | 8 | |
| KCh37-12 | 370 | 163 | 12 | |
| … | … | … | … | |
| KCH63-2 | 630 | 269 | 2 |
Silicon Si promotes the graphitization of cast iron and improves its casting properties. Gray cast iron contains 0.8 ... 4.5% Si.
Manganese Mn promotes the bleaching of cast iron, but a Mn content of up to 1.2% is beneficial because the hardness and strength of cast iron increases.
Phosphorus P increases the fluidity of cast iron, so its content up to 0.4% is permissible, but critical cast iron castings contain less than 0.15% phosphorus, because As its content increases, the fragility of cast iron increases.
Sulfur S makes graphitization difficult, increases brittleness and impairs the fluidity of cast iron, so sulfur in cast iron should be no more than 0.1%.
Gray cast irons are divided into modified, high-strength and malleable (Table 2).
In gray cast irons, graphite has a lamellar shape, in high-strength cast irons it has a spherical shape, and in malleable cast irons it has a flaky shape. Examples of designation of cast irons:
The formation of the cast iron structure occurs when the casting solidifies. The main factors influencing the structure formation of cast iron are its chemical composition (see table below) and the cooling rate of the casting in the mold.
Table 2 - The influence of chemical elements on the properties of cast iron
| Gray cast iron | Ductile iron | Malleable iron |
| Carbon | ||
| Increased carbon content leads to a decrease in strength, hardness and an increase in ductility; carbon improves the casting properties of cast iron | Increased carbon content improves the casting properties of cast iron | Carbon is the main regulator of the mechanical properties of malleable cast iron; cast iron has low fluidity and requires high superheat |
| Silicon | ||
| Silicon (taking into account the carbon content) promotes the release of graphite and reduces hardness, and also reduces shrinkage; increased silicon content reduces ductility and slightly increases hardness | With increasing silicon content, the tensile strength increases; with a further increase in the content, the tensile strength and relative elongation decrease | For ferritic malleable cast iron, the total silicon and carbon content should be 3.7-4.1%. The silicon content depends on the amount of carbon and wall thickness. With a silicon content of up to 1.5%, the mechanical properties of the alloy increase |
| Manganese | ||
| Manganese inhibits the release of graphite, promotes the crushing of perlite and the bleaching of cast iron; interacting with sulfur, neutralizes its harmful effects. The mechanical properties of cast iron increase with a manganese content of up to 0.7-1.3%, and with a further increase they decrease. Manganese increases alloy shrinkage | With increasing manganese content, the proportion of ferrite decreases and the amount of pearlite increases; at the same time, the tensile strength increases and the relative elongation decreases. To increase wear resistance, the manganese content is increased to 1.0-1.3% | Manganese increases the amount of fixed carbon and increases the strength of ferrite. When the manganese content increases to 0.8-1.4%, the amount of pearlite increases, the strength of the alloy increases, but ductility and toughness sharply decrease. In ferritic cast iron, the manganese content should not exceed 0.6%, in pearlitic cast iron - 1.0% |
| Magnesium | ||
| — | To form spherical graphite, the magnesium content must be at least 0.03%, and cerium at least 0.02% (residual content). At lower contents, not all graphite becomes spherical; part of it is contained in the form of plates, which reduces the mechanical properties of the alloy. With an increased content of magnesium (and cerium) in the structure of the alloy, cementite is formed and, consequently, the mechanical properties are reduced. The optimal content of residual magnesium is 0.04-0.08% | — |
| Sulfur | ||
| Sulfur reduces strength and ductility, but slightly increases the wear resistance of the alloy; it is considered a harmful impurity; it gives cast iron red brittleness (formation of cracks at high temperatures), and prevents the release of graphite. | The higher the sulfur content in the original cast iron, the more difficult it is to obtain a completely spherical graphite shape and, therefore, high mechanical properties | The sulfur content of ferritic ductile iron modified with aluminum can be increased to 0.2%; in this case, the mechanical properties increase due to the improvement of the graphite shape. The determining influence on the mechanical properties of cast iron is the ratio of manganese and sulfur content, which should be in the range of 0.8-3.0 |
| Phosphorus | ||
| Phosphorus has little effect on the process of carbon graphitization, but increases the fluidity of the alloy and gives cast iron cold brittleness, i.e., brittleness | Phosphorus has a significant effect on the structure and mechanical properties. To obtain cast iron with high ductility, the phosphorus content should not exceed 0.08%. To obtain cast iron with low ductility, the phosphorus content is increased to 0.12-0.15% | Phosphorus has the same effect on the structure and mechanical properties of the alloy as for gray cast iron. |
| Nickel | ||
| Nickel is an alloying element that has a beneficial effect on equalizing mechanical properties in castings with different wall thicknesses and increases hardness by 10 HB. With increasing nickel content, corrosion resistance increases and the alloy's machinability improves. | Nickel affects the thermal and electrical conductivity, as well as the corrosion resistance and heat resistance of the alloy. These properties increase with increasing nickel content. | Nickel promotes graphitization of carbon and increases the amount of pearlite in the metal base of the alloy |
| Chromium | ||
| Chromium is a carbide-forming element. With an increase in chromium, the strength and hardness of castings increases, and the process of carbon graphitization slows down. | With an increase in chromium content within certain limits, the heat resistance, corrosion resistance and wear resistance of the alloy increase | Chromium slows down the process of carbon graphitization. The chromium content in the alloy does not exceed 0.06-0.08%; increasing the content to 0.1 -0.12% leads to the formation of stable carbides in the alloy structure |
| Molybdenum | ||
| Molybdenum is an alloying element; slows down the process of carbon graphitization and promotes carbide formation. With increasing molybdenum content, hardness increases without compromising machinability and wear resistance increases | — | Molybdenum promotes the grinding of pearlite and graphite inclusions, increases the tensile strength by 3-7 kgf/mm2 with a molybdenum content of 0.5%; slows down the process of carbon graphitization |
| Copper | ||
| Copper promotes graphitization of carbon, increases fluidity, increases the strength and hardness of the alloy | When the alloy contains 1% copper, the tensile strength increases to 40%, and the fluidity increases to 50%, and, accordingly, with 2% copper - up to 65% and up to 70%. A copper content of more than 2% prevents the formation of spherical graphite in the alloy structure | Copper promotes carbon graphitization and increases the pearlite content in the alloy |
Small amounts of a variety of elements can enter the cast iron and have a noticeable effect on the structure and properties of the castings. Some of these elements are added on purpose, while others are impurities introduced into the metal from the charge. Some of these elements have positive effects, especially in gray cast iron, while others have negative effects and should be avoided in the melt. The table lists common sources of these elements, commonly found levels, and the main effects on cast iron. The results of using some elements as the main alloying agents (for example, chromium) are not indicated in the table.
| Element | Regular source | Normal content (%) | Impact on cast iron |
| Aluminum Al | Steel scrap, deoxidized Al, modifiers, ferroalloys, aluminum additives | Up to 0.03 | Promotes the formation of hydrogen gas pores in thin sections with an Al content above 0.005%. Neutralizes nitrogen. Promotes the formation of dross. When Al exceeds 0.08%, it has a negative effect on the shape of spherical graphite inclusions. Can be neutralized by cerium. Strong graphite stabilizer. |
| Antimony Sb | Steel scrap, enamelled scrap, bearing housings, antimony additives | Up to 0.02 | Strong stabilizer of perlite and carbides. Prevents the formation of spherical graphite in the absence of rare earth metals. |
| Arsenic As | Cast iron, steel scrap | Up to 0.05 | Strong stabilizer of perlite and carbides. Improves the shape of spherical graphite. |
| Barium Ba | Modifiers with barium | Up to 0.003 | Enhances the formation of graphitization centers of graphite and increases the duration of action of the modifier. Reduces the tendency to bleach and promotes the formation of graphite. |
| Bismuth Bi | Special additives, mold coating containing bismuth | Over 0.01 | Promotes the formation of chill and undesirable forms of graphite. Increases the number of inclusions of spherical graphite in HF containing rare earth metals (cerium). An excessive number of spherical graphite inclusions can cause shrinkage. |
| Boron B | Enameled scrap, special additives (for example, FeB). | Up to 0.01 | Above 0.001% promotes the formation of carbides especially in HF. 0.002% B improves the annealing ability of malleable cast iron. |
| Calcium Ca | Ferroalloys, modifiers | Up to 0.01 | Improves the degree of sphericity of graphite inclusions. Reduces the tendency to bleach and promotes the formation of graphite. |
| Cerium Ce | Most magnesium alloys, mischmetal or other rare earth sources | Up to 0.02 | Generally not used in gray cast iron. Suppresses the negative effects of unwanted elements in HF. Improves the degree of sphericity of graphite. Carbide stabilizer due to segregation. |
| Chrome Cr | Chrome alloy steel, some cast irons, ferrochrome | Up to 0.3 | Promotes the formation of bleach and perlite. Increases strength. Forms accumulations of carbides in HF at contents above 0.05%. |
| Cobalt Co | Tool steel | Up to 0.02 | Does not have a significant effect on cast iron. |
| Copper Cu | Copper wire, copper-based alloys, steel scrap, special copper additives. | Up to 0.5 | Promotes the formation of perlite. Increases strength. Reduces the process of ferritization in HF. No harmful effects. |
| Hydrogen H | Raw refractories, mold materials and wet additives. | — | Forms subsurface gas pores. To a small extent contributes to the formation of bleach. Promotes bleaching when there is a lack of manganese to neutralize sulfur. Promotes the formation of large graphite inclusions. |
| Lead Pb | Old paints, some types of enamels, automatic steel, solder, deposits on a gasoline engine. | Up to 0.005 | Promotes the formation of undesirable graphite structures in gray cast iron and significantly reduces strength at levels > 0.004%. Promotes the formation of pearlite and carbides. Causes the formation of degenerative forms of spherical graphite inclusions. The negative effect on graphite in high frequency is neutralized by rare earth metals (cerium). |
| Magnesium Mg | Additives containing magnesium modifiers. | 0,03 — 0,08 | Promotes the formation of spherical graphite inclusions and stabilizes carbides in HF. Not used in gray cast iron. |
| Manganese Mn | Most cast iron, scrap steel, additions of lump or briquetted ferromanganese. | 0,2 — 1,0 | Neutralizes sulfur, forming MnS. Promotes the formation of perlite. Forms carbide accumulations in the HF. At high contents it promotes the formation of gas pores in combination with high sulfur content. |
| Molybdenum Mo | Refined cast iron, alloy steel, ferromolybdenum additives | Up to 0.1 | Promotes the formation of perlite. Increases strength. May promote shrinkage and carbide formation. |
| Nickel Ni | Nickel-plated sheets, scrap steel, special cast irons. Ni/Mg alloy | Up to 0.5 | In small quantities there is little effect on the melt. Graphitizing effect in large quantities. |
| Nitrogen N | Coke, carburizers, binders, steel scrap, nitrided ferromanganese additives. | Up to 0.015 | Promotes the formation of compact graphite structures. Promotes the formation of perlite. Increases strength. High content leads to the formation of cracks in thick sections. Can be neutralized by Al, Ti and Zr. Has a negligible effect on HF. |
| Phosphorus P | Phosphorous cast iron and scrap, FeP additives. | Up to 0.1 | Increases carbon equivalent. Increases fluidity. Forms phosphide eutectic. Has a negative effect on HF at contents > 0.05%. At contents > 0.04% it causes scorching. |
| Silicon Si | Ferrosilicon alloys, scrap steel, cast iron. | 0,8-4,0 | Promotes graphitization, reduces chill, stabilizes ferrite, improves casting properties. |
| Sulfur S | Coke, carburizers, cast iron, cast iron scrap, iron sulfide additives. | Up to 0.15 (gray cast iron) | Has a strong negative effect on structures and properties if not balanced with manganese. Increases the sensitivity of the midrange to modification. May require an increase in Mg weights in HF. The sulfur content in HF should not exceed 0.03%. |
| Strontium Sr | Strontium containing modifiers | Up to 0.003 | Promote the formation of graphite in the midrange and high frequency. Significantly reduces chill in gray cast iron. |
| Tellurium Te | Automatic copper, mold coatings, residues from samples during thermal analysis. | Up to 0.003 | Strong carbide stabilizer. Causes the formation of many undesirable forms of graphite. The effect of Te is expressed at a content of 0.0003%. The influence is reduced when Te is combined with Mg and Ce in HF |
| Tin Sn | Solder, scrap tin, bronze components, tin additives. | Up to 0.15 | Significantly promotes the formation of perlite. Increases strength. HF becomes embrittled at contents > 0.08%. No other harmful effects were noted. |
| Titan Ti | Some cast irons, some paints and enamels, return of CVG, additions of titanium and ferrotitanium. | Up to 0.10 | Neutralizes nitrogen in gray cast iron. Causes the formation of hydrogen porosity in the presence of aluminum. Causes the formation of supercooled graphite in gray cast iron. Suppresses the formation of spherical graphite inclusions during the production of carbon dioxide. |
| Tungsten W | High speed tool steel | Up to 0.05 | Rarely present in significant quantities. Medium strength perlite stabilizer. |
| Vanadium V | Scrap, tool steel, some cast iron, ferrovanadium additives. | Up to 0.10 | Causes the formation of whitewash. Grinds flake graphite inclusions. Significantly increases strength. |
Cast iron marking
Products made of malleable cast iron are marked KCH and subsequent numbers.
The first pair of numbers is the average tensile strength (tensile strength), reduced by an order of magnitude, and the second is the percentage of elongation. For example, a product of the KCh 30-6 brand has a temporary tensile strength σв = 294 N/mm2, and a relative elongation - δ = 6%. According to GOST 1215–79, 11 types of malleable cast iron are defined.
The table shows the mechanical characteristics of different brands of products.
Areas of use
Malleable cast iron is intended for use:
- in the mechanical engineering industry for the manufacture of machine tool structures;
- for the manufacture of car bodies and components;
- in the production of railway cars;
- in the manufacture of equipment for agriculture.
Despite the fact that pearlitic cast iron has better characteristics, ferritic castings are mainly used, since their production is cheaper.
Pearlitic castings are used in the production of parts subject to increased loads. For example, they are used to produce automobile springs, components for diesel and other engines, etc.
With many technological advantages, ductile iron is mainly used for castings with relatively thin walls ranging from 3 mm to 40 mm.