Content
- 1 Historical information
- 2 Origin of the name
- 3 Being in nature
- 4 Receipt 4.1 Worldwide production
- 7.1 Lead halides
- 8.1 Abundance of lead isotopes
- 9.1 In medicine
Lead Ingots
Environmental characteristics
Lead pollution of the environment is considered one of the most dangerous. All products that use lead require special disposal, which is carried out only by licensed services.
Unfortunately, lead pollution is caused not only by the activities of enterprises, where it is at least regulated. In city air, the presence of lead vapor ensures the combustion of fuel in cars. Against this background, the presence of lead stabilizers in such familiar structures as a metal-plastic window no longer seems worth attention.
Lead is a metal of industrial importance. Despite its toxicity, it is used too widely in the national economy for the metal to be replaced with anything.
This video will tell you about the properties of lead salts:
Historical information
Lead has been used for thousands of years because it is widespread and easy to mine and process. It is very malleable and melts easily. Lead smelting was the first metallurgical process known to man. Lead beads dating back to 6400 BC. BC, were found in the Çatalhöyük culture. The oldest object made of lead is often considered to be a figurine of a standing woman in a long skirt from the First Dynasty of Egypt, dating back to 3100-2900. BC BC, stored in the British Museum (stock number EA 32138). It was found in the Temple of Osiris at Abydos and brought from Egypt in 1899. In Ancient Egypt, lead medallions were used. In the Early Bronze Age, lead was used along with antimony and arsenic. Lead is referred to as a specific metal in the Old Testament.
Lead pipes of an ancient Roman water supply with inscriptions
The largest producer of lead in the pre-industrial era was Ancient Rome, with an annual production of 80,000 tons. Roman mining of lead took place in Central Europe, Roman Britain, the Balkans, Greece, Asia Minor and Spain. The Romans widely used lead in the production of pipes for water supply systems, and lead pipes often bore the inscriptions of Roman emperors. True, even Pliny and Vitruvius believed that this was not good for public health.
Papal bull of 1637 with lead seal
After the fall of the Roman Empire in the 5th century. n. e. Lead use in Europe fell and remained low for about 600 years. Then lead began to be mined in eastern Germany. Lead sugar has been added to wine since Roman times to improve its taste; this became widespread and continued even after the ban by a papal bull in 1498. This use of lead in the Middle Ages led to epidemics of lead colic. In Ancient Rus', lead was used to cover the roofs of churches, and was also widely used as a material for hanging seals on letters. Later, in 1633, a water supply system with lead pipes was built in the Kremlin, through which water came from the Vodovzvodnaya Tower; it existed until 1737.
In alchemy, lead was associated with the planet Saturn and was represented by its symbol ♄. In ancient times, tin, lead and antimony were often not distinguished from each other, considering them to be different types of the same metal, although Pliny the Elder distinguished between tin and lead, calling tin “plumbum album” and lead “plumbum nigrum”.
The Industrial Revolution led to a new increase in the demand for lead. By the beginning of the 1840s. annual production of refined lead exceeded 100,000 tons for the first time and grew to over 250,000 tons over the next 20 years. Until the last decades of the 19th century, lead mining was primarily carried out by three countries: Britain, Germany and Spain. By the beginning of the 20th century, lead mining in Europe had become less than in the rest of the world, thanks to increased production in the United States, Canada, Mexico and Australia. Until 1990, large quantities of lead were used (together with antimony and tin) to cast typographical fonts, and also in the form of tetraethyl lead to increase the octane number of motor fuels.
From nuclear energy to fishing pleasures
Lead is widely used in industry and everyday life.
Areas of application of lead
In medicine, X-ray studies are indispensable without metal covers.
In geology, a “heavy” metal is used to determine the age of the Earth and in particular its minerals and rocks. These are geochronological methods using isotopes of various elements, including lead.
We recommend: CESIUM - a metal with an explosive character
If all lead-acid batteries disappeared tomorrow, we would have to switch to horses, bicycles or walk. Lead powers every car on the planet.
He, our gray hero, is included in soldering compounds. And try to prove to the fisherman that he can somehow manage without a lead sinker.
How metal became a murder weapon
It seems that humanity cannot live without wars, fights, showdowns, or at least hunting. The hobby of the strong half of humanity has always meant close contact with lead. Lead is easy to process. Even in ancient Rome, sling shells were cast from lead. It was later used to make bullets for centuries. To this day, bullets are made from lead for one simple reason - great penetration relative to the size and speed of the bullet.
In the first firearms, bullets were simply lead balls; now they are perfectly balanced little killers of different designs. But whether it is ordinary or special (armor-piercing, expansive, explosive), the bullet contains lead. Bullet cores are made of lead wire (for 45 caliber the wire is thicker, for 20 caliber it will be thinner). The cores are “dressed” in copper sleeves.
Lead nitrates, perchlorates and azides are used in the preparation of explosives.
Metal's dark past
Pliny and the physician Dioscorides wrote about the toxic properties of metal (they were concerned about the lead water pipes and metal tiles that lined the walls and sometimes the floor).
Russian Tsar Alexei Mikhailovich, while reconstructing the Kremlin, ordered the installation of a machine on the Vodovzvodnaya Tower that raised water into the Kremlin. Everything was fine, except the pipes were lead. Some historians and doctors blame the impact of the metal on the king’s offspring. His children, Fedor and Ivan, were very sick. Drinking lead water also affected Peter the Great. In the movies, the Tsar Father was handsome, but in life he was awkward, with narrow shoulders, a wide pelvis, long arms and legs.
Poisoning with toxic metal compounds continued further.
TPP (tetraethyl lead)
Our love for four wheels and speed led to the development of this substance. More precisely, to its addition to gasoline, which began to be called “leaded.” The cars ran faster on this kind of gasoline, and there were more and more of them. Things were heading towards a geochemical catastrophe - the thermal power plant was in the ground, in the air, in human bodies. The governments of the countries realized that at this rate, with lead poisoning, there will soon be no one to rule. And they introduced a ban on leaded gasoline.
Related publications
- PLATINUM - “rotten silver” or noble metal
- BRONZE - an alloy for all times and peoples
- BISMUT - radioactive and safe
- TANTALUM - hard, rare and expensive
origin of name
The origin of the word "lead" is unclear. This metal is called “tin” in Bulgarian; in most other Slavic languages (Serbo-Croatian, Czech, Polish, lead is called a word similar in sound to “tin”: volava
,
olovo
,
ołów
, etc. A word with the same meaning, but similar in pronunciation to “lead”, is found in the languages of the Baltic group:
švinas
(Lithuanian),
svins
(Latvian), as well as in several Slavic languages - Russian, Ukrainian (
lead
), Belarusian (
svinec
) and Slovenian (
svinec
).
Latin plumbum
gave the English word
plumber
- plumber (in Ancient Rome, water pipes were made of this particular metal, as the most suitable for casting), and the name of the Venetian prison with a lead roof - Piombi, from which, according to some sources, Casanova managed to escape.
Being in nature
The content in the earth's crust is 1.6·10−3% by mass. Native lead is rare; the range of rocks in which it is found is quite wide: from sedimentary rocks to ultramafic intrusive rocks. In these formations it often forms intermetallic compounds (for example, zvyagintsevite (Pd,Pt)3(Pb,Sn), etc.) and alloys with other elements (for example, (Pb + Sn + Sb)). It is part of 80 different minerals. The most important of them are: galena PbS, cerussite PbCO3, anglesite PbSO4 (lead sulfate); of the more complex ones - tillite PbSnS2 and betechtinite Pb2(Cu,Fe)21S15, as well as lead sulfosalts - jamesonite FePb4Sn6S14, boulangerite Pb5Sb4S11. Always found in uranium and thorium ores, often having a radiogenic nature. Under natural conditions, it often forms large deposits of lead-zinc or polymetallic ores of the stratiform type (Kholodninskoye, Transbaikalia), as well as skarn (Dalnegorskoye (formerly Tetyukhinskoye), Primorye; Broken Hill in Australia) type; galena is often found in deposits of other metals: pyrite-polymetallic (Southern and Middle Urals), copper-nickel (Norilsk), uranium (Kazakhstan), gold ore, etc. Sulfosalts are usually found in low-temperature hydrothermal deposits with antimony, arsenic, and also in gold deposits (Darasun, Transbaikalia). Lead minerals of the sulfide type have a hydrothermal genesis, minerals of the oxide type are common in weathering crusts (oxidation zones) of lead-zinc deposits. Lead is present in clarke concentrations in almost all rocks. The only place on earth where rocks contain more lead than uranium is the Kohistan-Ladakh arc in northern Pakistan.
Galena, Dalnegorsk skarn deposit
The table shows some parameters of the prevalence of lead in natural conditions according to A.P. Vinogradov:
| Breeds | Stone meteorites | Dunits and others. | Basalts, etc. | Diorites, etc. | Granites, etc. | Glina, etc. | Earth's crust |
| Content, wt.% | 0000002×10−5 | 0001×10−5 | 0008×10−4 | 0001,5×10−3 | 0002×10−3 | 0002×10−3 | 1,6×10−3 |
| Objects | Living matter of the Earth | Lithosphere | Soil0 | Plants (in ash) | Ocean water (mg/l) |
| Content, wt.% | 000000005×10−5 | 000,0016 | 00,001 | 000000,001 | 0000000,00003 |
Generalized concentrations of elements in minerals are given in the table; in parentheses are the amounts of minerals for which the average contents of components are calculated.
| Mineral | Lead (general) | Uranus | Thorium |
| 00Bittersweet | 04,750 (308) | 58,87 (242) | 2,264 (108) |
| 00Monazite | 00,6134 (143) | 0,2619 (160) | 6,567 (150) |
| 000Ortit | 00,0907 (90) | 0,1154 (88) | 6,197 (88) |
| 000Zircon | 00,0293 (203) | 0,1012 (290) | 0,1471 (194) |
| Sphene (Titanit) | 00,0158 (12) | 0,0511 (14) | 0,0295 (21) |
Receipt
To obtain lead, ores containing galena are mainly used. First, a concentrate containing 40-70 percent lead is obtained by flotation. Then, several methods are possible for processing the concentrate into werkbley (blank lead): the formerly widespread method of mine reduction smelting, the method of oxygen-suspended cyclone electrothermal smelting of lead-zinc products (KIVTSET-TSS), the Vanyukov smelting method (melting in a liquid bath) developed in the USSR. . For smelting in a shaft (water jacket) furnace, the concentrate is first sintered and then loaded into a shaft furnace, where lead is reduced from the oxide.
Werkbley, containing more than 90 percent lead, undergoes further purification. First, zeigerization and subsequent sulfur treatment are used to remove copper. Arsenic and antimony are then removed by alkaline refining. Next, silver and gold are isolated using zinc foam and the zinc is distilled off. Bismuth is removed by treatment with calcium and magnesium. As a result, the impurity content drops to less than 0.2%.
World production
Countries are the largest producers of lead (including secondary lead) in 2004 (according to ILZSG):
| A country | Quantity in metric kilotons |
| European Union | 2200 |
| USA | 1400 |
| China | 1200 |
| Russia | 1100 |
| South Korea | 600 |
| Kazakhstan | 550 |
| Ukraine | 400 |
Mining and production
According to the US Geological Bureau, the world's metal reserves reach one and a half billion tons. The main part of them is owned by the following countries:
- China;
- USA;
- Russia;
- SOUTH AFRICA;
- Peru;
- Canada;
- Mexico.
The annual production of metal in the world is about 3 million tons. China is creating a lead shortage in this market. Its demand for the metal accounts for about 45% of global production.
Our gray hero is produced from minerals and recycled materials (tons of batteries from used cars).
Physical properties
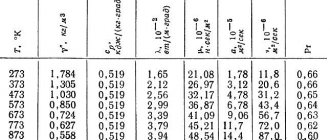
Lead has a rather low thermal conductivity, it is 35.1 W/(m K) at a temperature of 0 °C.
The metal is soft, can be cut with a knife, and is easily scratched with a fingernail. On the surface it is usually covered with a more or less thick film of oxides; when cut, a shiny surface is revealed, which fades over time in air. Melting point - 600.61 K (327.46 °C), boils at 2022 K (1749 °C). Belongs to the group of heavy metals; its density is 11.3415 g/cm3 (at +20 °C). As temperature increases, lead density decreases: Change in lead density depending on temperature
| Temperature, °C | Density, g/cm3 |
| 327,6 | 10,686 |
| 450 | 10,536 |
| 650 | 10,302 |
| 850 | 10,078 |
Tensile strength - 12-13 MPa (MN/m2).
At a temperature of 7.26 K it becomes a superconductor.
Chemical properties
Electronic formula: 5s25p65d106s26p2, ionization energy (Pb → Pb+ + e−) is 7.42 eV. There are 4 unpaired electrons on the outer electron shell (2 on the p- and 2 on the d-sublevels), therefore the main oxidation states of the lead atom are +2 and +4.
- Salts of divalent lead react with alkalis, forming almost insoluble lead hydroxide:
Pb2+ + 2OH− = Pb(OH)2
- If there is an excess of alkali, the hydroxide dissolves:
Pb(OH)2 + 2OH− = [Pb(OH)4]2−
- Reacts with alkalis and acids:
Pb + 2NaOH + 2H2O = Na2[Pb(OH)4] + H2↑ Pb + 4HNO3 = Pb(NO3)2 + 2NO2↑ + 2H2O Pb + 2HCl = PbCl2 + H2↑
Lead forms complex compounds with a coordination number of 4, for example, [Pb(OH)4]2−
The disproportionation reaction between PbO2 and Pb underlies the operation of lead batteries.
Lead Babbitts
These are alloys of lead in order to give it better wear resistance and reduce the coefficient of friction. A number of metals can be used as alloying additives - copper, nickel, calcium and others. Lead-based babbits have proven themselves in the production of bearings for various purposes:
- carriages and electric locomotives;
- rotating parts of diesel engines;
- heavy industry;
- complex technology and automotive industry.
Since 1847, lead babbits have been an integral part of Russian industry. Among the disadvantages, quite rapid destruction from heavy loads is noted, so the durability of units using such alloys strongly depends on the quality of the metal of the bearing housing. The thicker and stronger it is, the more voluminous the babbitt layer can be made, and therefore increase the service life of the product.
Main lead compounds
Main article: Category:Lead compounds
Lead in compounds can be in the +2 and +4 oxidation states, forming the compounds Pb(II) and Pb(IV), respectively. In both oxidation states, lead is amphoteric and can either act as cations Pb2+ and Pb4+, or be part of anions (plumbite PbO2- 2 with Pb(II) and plumbates with Pb(IV): metaplumbate PbO2- 3 and orthoplumbate PbO4- 4), and therefore can form four types of salts.
Lead halides
Lead forms halides in the +2 oxidation state of the form PbHal2 for all halogens. Lead(IV) halides are also known: PbF4 and PbCl4, tetrabromides and tetraiodides have not been obtained.
- Lead(II) fluoride
- Lead(II) chloride is a white crystalline powder, soluble in hot water. It also dissolves well in solutions of other chlorides, especially ammonium chloride NH4Cl.
- Lead(II) bromide
- Lead(II) iodide
Lead chalcogenides
Lead chalcogenides - lead sulfide PbS, lead(II) selenide PbSe and lead telluride PbTe - are black crystals that are narrow-gap semiconductors.
Lead oxides
Main article: Lead oxides
Lead oxides are predominantly basic or amphoteric in nature. Many of them are painted red, yellow, black, and brown. In the photograph at the beginning of the article, on the surface of the lead casting, tarnish colors are visible in its center - this is a thin film of lead oxides formed due to the oxidation of hot metal in air. Lead forms two simple oxides - lead(II) oxide PbO and lead(IV) oxide PbO2 - and one mixed oxide Pb3O4 (red lead), which is actually plumbate (IV) lead(II) Pb2PbO4.
Lead salts
- Lead(II) sulfate PbSO4
- Lead(II) nitrate Pb(NO3)2
- Lead(II) acetate Pb(CH3COO)2 ( lead sugar
). - Lead(II) chromate PbCrO4
Plumbates
Calcium orthoplumbate is a faint orange crystalline substance. Throughout the 20th century it was used in chemistry and medicine as a source for easily producing pure oxygen, which is released when heated. This was also facilitated by the ease of synthesis of the substance itself - it is obtained by calcining lead oxide and calcium carbonate. Further success was hampered only by the toxicity of the process.
The following group of plumbates is used in the chemical production of complex lead compounds:
- sodium metaplumbate;
- ammonium hexachloroplumbate;
- hexachloroval acid;
- potassium triodoplumbate.
All of them easily decompose, dissolve in water and react with other compounds. This makes them an indispensable raw material and catalyst for many processes, as well as a basic component in the production of some complex substances.
Isotopes
Main article: Isotopes of lead
All lead is mainly a mixture of isotopes 204Pb, 206Pb, 207Pb, 208Pb. These isotopes are not radioactive, that is, they are stable. Lead is the last element in the periodic table for which stable isotopes exist; elements after lead do not have stable isotopes (although bismuth-209 can in practice be considered stable, since its half-life is about a billion times greater than the age of the Universe). The isotopes 206Pb, 207Pb, 208Pb are radiogenic and are formed as a result of the radioactive decay of 238U, 235U and 232Th, respectively. The isotope 208 82Pb126 is one of the five doubly magic nuclei existing in nature. Radioactive decay schemes look like:
238U → 206Pb + 84He; 235U → 207Pb + 74He; 232Th → 208Pb + 64He.
The decay equations look like this:
206Pb =238 U (eλ8t − 1 ), 207Pb =235 U (eλ5t − 1 ), 208Pb =232 Th(eλ2t − 1 ),
where 238U, 235U, 232Th are modern isotope concentrations; λ8 = 1.55125 ⋅ 10−10 year−1, λ5 = 9.8485 ⋅ 10−10 year−1, λ2 = 4.9475 ⋅ 10−11 year−1 - decay constants of atoms of uranium 238U, uranium 235U and thorium, respectively 232Th.
In addition to these isotopes, unstable isotopes 194Pb - 203Pb, 205Pb, 209Pb - 214Pb are also known. Of these, the longest-lived are 202Pb and 205Pb (with half-lives of 52.5 thousand and 15.3 million years). The short-lived isotopes of lead 210Pb (radium D), 211Pb (actinium B), 212Pb (thorium B) and 214Pb (radium B) have half-lives of 22.2 years, 36.1 minutes, 10.64 hours and 26.8 minutes, respectively ( rarely used historical names of these isotopes are given in parentheses); these four radioactive isotopes are part of the radioactive series of uranium and thorium and, therefore, also occur in nature, although in extremely small quantities.
The number of nuclei of the 204Pb isotope (non-radiogenic and non-radioactive) is stable; in lead minerals, the concentration of 204Pb largely depends on the concentration of radiogenic isotopes formed both during the decay of radioactive nuclei and in the processes of secondary transformation of lead-containing minerals. Since the number of radiogenic nuclei formed as a result of radioactive decay depends on time, both absolute and relative concentrations depend on the time of formation of the mineral. This property is used to determine the age of rocks and minerals.