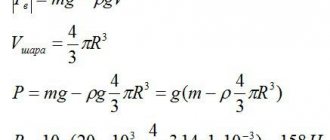4.7
Average rating: 4.7
Total ratings received: 115.
4.7
Average rating: 4.7
Total ratings received: 115.
In physics, melting is the transition of a substance from a solid to a liquid state. Classic examples of the melting process are the melting of ice and the transformation of a solid piece of tin into liquid solder when heated with a soldering iron. The transfer of a certain amount of heat to a body leads to a change in its state of aggregation.
Why does a solid become liquid?
Heating a solid body leads to an increase in the kinetic energy of atoms and molecules, which at normal temperatures are located clearly at the nodes of the crystal lattice, which allows the body to maintain a constant shape and size. When certain critical speed values are reached, atoms and molecules begin to leave their places, bonds are broken, the body begins to lose its shape - it becomes liquid. The melting process does not occur abruptly, but gradually, so that for some time the solid and liquid components (phases) are in equilibrium. Melting refers to endothermic processes, that is, those that occur with the absorption of heat. The opposite process, when a liquid solidifies, is called crystallization.
Rice. 1. Transition of the solid, crystalline state of a substance into the liquid phase.
It was discovered that until the end of the melting process the temperature does not change, although heat is constantly supplied. There is no contradiction here, since the incoming energy during this period of time is spent on breaking the crystalline bonds of the lattice. After the destruction of all bonds, the influx of heat will increase the kinetic energy of the molecules, and consequently, the temperature will begin to rise.
Rice. 2. Graph of body temperature versus heating time.
Thematic assignments
The study of thermal phenomena and their features, which include specific heat, is included in the school curriculum in physics for high school. Thematic tasks are used to test the mastery of the material covered.
In addition to the usual text conditions, tasks to find the specific heat of vaporization are in most cases accompanied by graphs displaying the temperature changes that occurred with the substance as it absorbed heat.
But graphical tasks are not the most interesting. Some of the most entertaining tasks include:
- A piece of ice, placed at a temperature of -90 degrees Celsius, began to be heated by supplying constant thermal power to it. After 63 seconds from the start of heating, the ice reached the temperature required for melting. It is required to find the time in seconds that the process of melting ice will take from the moment it reaches the desired heating, provided that there is no loss of heat. Answer: 110 seconds.
- A piece of lead, which was at a temperature of +27.5 degrees Celsius, began to be heated by applying constant thermal power to it. 39 seconds after the start of heating, the temperature of the lead reached the melting level (+327.5 degrees Celsius). It is necessary to determine the duration of the lead melting process in seconds from this moment, taking into account the absence of heat losses. Answer: 25 seconds.
Comparing the answers to these problems allows us to estimate the difference between the specific melting values of ice and lead. In the first it is very large, and in the second, on the contrary, it is small. This is not surprising - the amount of heat required for melting directly depends on the properties and characteristics of the substance, in particular, on the energy of the bonds connecting the particles of this substance to each other.
The role of the large specific value that ice has is invaluable both for nature and for humanity. If this figure had been lower, then in the spring all the ice and snow would have melted, which would have had dire consequences. The streams of water resulting from such melting would wash away everything in its path.
Fortunately, ice and snow masses are not capable of melting in a few moments . The physical properties of these substances once again prove that nature is a brilliant and inimitable creator.
Determination of specific heat of fusion
The specific heat of fusion (designated by the Greek letter “lambda” - λ) is a physical quantity equal to the amount of heat (in joules) that must be transferred to a solid body weighing 1 kg in order to completely transform it into the liquid phase. The formula for the specific heat of fusion looks like this:
$$ λ ={Q \over m}$$
Where:
m is the mass of the melting substance;
Q is the amount of heat transferred to the substance during melting.
Values for different substances are determined experimentally.
Knowing λ, we can calculate the amount of heat that must be imparted to a body of mass m for its complete melting:
$$Q={λ*m}$$
Related quantities
The so-called specific indicators exist to characterize not only melting and crystallization. In physical science, in addition to these processes, specific heat values are characterized by:
- steam generation;
- condensation;
- heat capacity.
The specific heat of vaporization and condensation reflects the volume of heat required to convert a unit mass of liquid into vapor and vice versa. The formula for this quantity is: Q/m. So, in essence, it is the same as the enthalpy of melting and crystallization.
As for specific heat capacity, it is an indicator of the ratio of heat capacity and mass of a substance. It is equal to the volume of heat, the transfer of which to a unit mass of a substance is necessary to change its temperature by one degree.
Specific heat of fusion of certain substances
Information on specific heat values for a specific substance can be found in book reference books or in electronic versions on Internet resources. They are usually presented in table form:
Specific heat of fusion of substances
| Substance | 105 * J/kg | kcal/kg | Substance | 105 * J/kg | kcal/kg |
| Aluminum | 3,8 | 92 | Mercury | 0,1 | 3,0 |
| Iron | 2,7 | 65 | Lead | 0,3 | 6,0 |
| Ice | 3,3 | 80 | Silver | 0,87 | 21 |
| Copper | 1,8 | 42 | Steel | 0,8 | 20 |
| Naphthalene | 1,5 | 36 | Zinc | 1,2 | 28 |
| Tin | 0,58 | 14 | Platinum | 1,01 | 24,1 |
| Paraffin | 1,5 | 35 | Gold | 0,66 | 15,8 |
One of the most refractory substances is tantalum carbide - TaC. It melts at a temperature of 39900C. TAC coatings are used to protect metal molds in which aluminum parts are cast.
Rice. 3. Metal melting process.
Change in internal energy and temperature during melting
So where does the energy that we impart to the body go during melting?
You know that in crystalline solids, atoms (or molecules) are arranged in a strict order (Figure 1). They do not move as actively as in gases or liquids. However, they are also in thermal motion - oscillating.
Figure 1. Structure of crystalline quartz
Take another look at the ice melting and solidifying graph (Figure 2).
Figure 2. Graph of ice melting and solidification
The ice is heated in section AB. At this time, the average speed of movement of its molecules increases. This means that their average kinetic energy and temperature also increase. The range of vibrations of atoms (or molecules) increases.
This happens until the heated body reaches its melting point.
At the melting temperature, the order in the arrangement of particles in crystals is disrupted.
This is how the substance begins the transition from solid to liquid.
This means that the energy that the body receives after reaching the melting temperature is spent on the destruction of the crystal lattice. Therefore, body temperature does not increase - part of the BC graph.
Exercise 12
1. Figure 19 shows graphs of temperature versus time for two bodies of the same mass. Which body has a higher melting point? Which body has a higher specific heat of fusion? Are the specific heat capacities of the bodies the same?
2. Melting ice was brought into a room whose temperature was about °C. Will the ice in this room continue to melt?
3. Pieces of ice are floating in a bucket of water. The total temperature of water and ice is about °C. Will the ice melt or will the water freeze? What does this depend on?
4. How much energy does it take to melt ice weighing 4 kg at a temperature of 0 °C?
5. How much energy is required to melt 20 kg of lead at its melting temperature? How much energy will be needed for this if the initial temperature of the lead is 27 °C?
Application of metal in industrial production
Under natural conditions, aluminum tends to form a thin oxide film, which prevents reactions with water and nitric acid (without heating). When the film is destroyed as a result of contact with alkalis, the chemical element acts as a reducing agent.
In order to prevent the formation of an oxide film, other metals (gallium, tin, indium) are added to the alloy. The metal is practically not subject to corrosion processes. It is a popular material in various industries.
Aluminum and its alloys are in great demand in various spheres of human life.
- Aluminum is considered a popular material for making tableware and the main raw material for the aviation and space industries. The excellent electrical conductivity of the metal allows it to be used for sputtering conductors in microelectronics.
- The property of aluminum and its alloys to become brittle at low temperatures allows it to be used in cryogenic technology. Reflectivity and low cost, ease of vacuum deposition make aluminum an indispensable material for the manufacture of mirrors.
- The application of metal to the surface of parts of turbines and oil platforms makes steel alloys resistant to corrosion. Metal sulfide is used to produce hydrogen sulfide, and pure aluminum is used as a reducing agent for rare alloys from oxides.
- The chemical element is used as a component of compounds, for example, in aluminum bronzes and magnesium alloys. Along with other materials, it is used for the manufacture of spirals in electric heating devices. Metal compounds are widely used in glass making.
- Currently, pure aluminum is rarely used as a material for jewelry, but its alloy with gold, which has a special shine and sparkle, is gaining popularity. In Japan, metal is used instead of silver to make jewelry.
- Aluminum is registered as an additive in the food industry. Aluminum beer cans have been a popular beverage packaging since the 1960s. The technological line provides for the production of containers of 0.33 and 0.5 liters. The packaging has the same diameter and differs only in height.
- The main advantage of packaging over glass is the possibility of recycling the material.
- Cans for beer (carbonated drinks) can withstand pressure up to 6 atmospheres, have a dome-shaped, thick bottom and thin walls. Features of the manufacturing technology by drawing ensure the structural strength and reliable performance properties of the container.
general information
A correct understanding of the specific heat of fusion is impossible without studying the key features of the melting process itself. Both during melting and during crystallization of a substance, its internal energy changes. During the first process, it increases, since it is invariably accompanied by heating - the main condition for increasing energy. The temperature during melting remains unchanged. In a certain sense, this is paradoxical, because internal energy can be characterized by temperature.
However, there is a very simple and logical explanation for the increase in energy at a constant temperature. During the melting process, the spatial lattice of the crystalline body is destroyed, and all the energy is spent on this. The destruction of the crystal lattice requires the expenditure of a certain amount of energy from some external source. As a consequence, during the melting process the internal energy of the body increases.
In the process of solidification of a body or, in other words, crystallization, on the contrary, its internal energy decreases, since it gives off heat to the bodies that surround it. Solidification (crystallization) is the reverse process in relation to melting. Molecules of a substance form a common (single) system, and during this unification, the excess energy given off by the components of the substance is absorbed by the external environment.
Basic information about the heat of fusion
According to the law of conservation of energy, a body absorbs during melting and gives off during solidification (at the temperature required for each of these processes) an equal amount of heat.
The heat of fusion is the amount of heat that is necessary for a physical body at the melting point to transform into a liquid state from a solid. This thermal phenomenon is a special case of a phase transition in thermodynamics.
The heat of melting is influenced by the mass of the melting substance, as well as the properties that it possesses and which are characteristic of it. This connection between the heat of melting of a physical body and the type of substance, expressed through the dependence of the first on the second, is measured by a specific value.
Melting a substance requires the same amount of heat that is released during crystallization, therefore, the definition of the specific value of heat exists in two equivalent concepts - for melting and for crystallization. This quantity also has an alternative name - enthalpy of fusion.
Measurement Features
Physicists have experimentally established that to transform the same substance into a liquid from a solid state, different amounts of heat are required . Then the experimental researchers decided to compare these indicators at the same mass of the substance. This is how the concept of specific value appeared.
According to its simplified definition, it shows the ratio of the heat of fusion of a body of a certain substance and its mass. This indicator is considered the main characteristic for both melting and crystallization.
The unit of measurement of this quantity, according to the International System of Units, is J/kg (joule per kilogram). The specific indicator is denoted by the letter lambda (less commonly read as lambda) from the Greek alphabet (analogous to the Cyrillic letter “l”).
The specific heat of fusion is found using the formula: lambda = Q/m, where Q is the designation of the amount of heat that the substance received during melting or released during crystallization, and m is the mass of the substance (melting or crystallizing). The absence of a temperature indicator in the dimension is due to the fact that the temperature does not change either during melting or during crystallization.
The specific value during melting is always positive, and during crystallization it is negative. The only exception to this rule exists (or rather, is known to science) - this is a chemical element of the periodic system called helium , which is under high pressure. It is negative when melted.
To convert a substance in the amount of one kilogram from a solid to a liquid state, you need to heat it to the melting point and add heat to it in an amount equal to the specific indicator. During the crystallization of one kilogram of a substance, heat is released in exactly the same amount.
To find the amount of heat required to melt or crystallize a substance at the appropriate temperatures, the specific value must be multiplied by the mass of the substance. For crystallizing bodies, this indicator will have a minus sign, that is, negative. This is due to the fact that during the hardening process all the heat is lost - released without being retained.
comparison table
A table with the specific heat of fusion of some substances and chemical elements (substances in the table are not arranged in alphabetical order, but by decreasing specific index):
| Name of substance or element | Specific heat of fusion in kJ/kg |
| Aluminum | 390 |
| Ice | 330 |
| Iron | 277 |
| Copper | 213 |
| Naphthalene | 151 |
| Paraffin | 150 |
| Ether | 113 |
| Zinc | 112 |
| Silver | 105 |
| Platinum | 101 |
| Gray cast iron | 100 |
| Steel | 83 |
| Gold | 66 |
| Tin | 61 |
| Lead | 25 |
| White cast iron | 14 |
| Mercury | 12 |
Specific values for these substances are considered tabular (constant and known) values, so there is no need to make calculations to find them.
What is heat characterized by?
Heat transfer implies an increase in the internal energy of some bodies and its decrease in other bodies. All this is accompanied by the absence of changes in mechanical actions and work. However, the internal energy of the heating object decreases, and the heated body increases.
As mentioned, the heat transfer process is determined by the amount of heat. This concept refers to the change in the internal energy of an object that occurs at the end of heat exchange. The calculation often uses a physical unit called a calorie.
A calorie is equal to the amount of heat required to warm a gram of water by 1° Celsius. The researchers found that to do this, you need to do 4.18 J of work. This means that a calorie is equal to 4.18 Joules.
Since heat equals the change in energy inside an object, we must say that c represents how much the energy of one kg of object changes when it is heated.
Boiling
Boiling is the formation of vapor that occurs throughout the entire volume of a liquid.
Boiling is possible because a certain amount of air is always dissolved in a liquid, which gets there as a result of diffusion. When the liquid is heated, this air expands, air bubbles gradually increase in size and become visible to the naked eye (in a pan of water they settle on the bottom and walls). Inside the air bubbles there is saturated steam, the pressure of which, as you remember, increases rapidly with increasing temperature.
The larger the bubbles become, the greater the Archimedean force acts on them, and at a certain moment the bubbles begin to separate and float up. Rising upward, the bubbles enter less heated layers of the liquid; the vapor in them condenses, and the bubbles shrink again. The collapse of the bubbles causes the familiar noise that precedes the boiling of the kettle. Finally, over time, the entire liquid warms up evenly, the bubbles reach the surface and burst, throwing out air and steam - the noise is replaced by gurgling, the liquid boils.
The bubbles thus serve as “conductors” of vapor from inside the liquid to its surface. During boiling, along with normal evaporation, the liquid is converted into steam throughout the entire volume - evaporation into air bubbles, followed by the release of steam outside. This is why boiling liquid evaporates very quickly: a kettle, from which the water would evaporate for many days, will boil away in half an hour.
Unlike evaporation, which occurs at any temperature, a liquid begins to boil only when it reaches its boiling point - precisely the temperature at which air bubbles are able to float and reach the surface. At the boiling point, the saturated vapor pressure becomes equal to the external pressure on the liquid (in particular, atmospheric pressure). Accordingly, the greater the external pressure, the higher the temperature at which boiling will begin.
At normal atmospheric pressure (atm or Pa), the boiling point of water is . Therefore, the pressure of saturated water vapor at temperature is equal to Pa. This fact is necessary to know to solve problems - it is often considered known by default.
At the top of Elbrus, the atmospheric pressure is atm, and water there will boil at a temperature of . And under pressure atm, water will begin to boil only at .
The boiling point (at normal atmospheric pressure) is a strictly defined value for a given liquid (boiling points given in the tables of textbooks and reference books are the boiling points of chemically pure liquids. The presence of impurities in a liquid can change the boiling point. For example, tap water contains dissolved chlorine and some salts, so its boiling point at normal atmospheric pressure may differ slightly from ). So, alcohol boils at , ether - at , mercury - at . Please note: the more volatile a liquid is, the lower its boiling point. In the table of boiling points we also see that oxygen boils at. This means that at normal temperatures oxygen is a gas!
We know that if the kettle is removed from the heat, the boiling will immediately stop - the boiling process requires a continuous supply of heat. At the same time, the temperature of the water in the kettle stops changing after boiling, remaining equal all the time. Where does the supplied heat go?
The situation is similar to the melting process: heat is used to increase the potential energy of the molecules. In this case, to perform work to remove molecules at such distances that the forces of attraction will be unable to keep the molecules close to each other, and the liquid will turn into a gaseous state.
Peculiarities
Along with the great variety of known metals, the element in question has a relatively small thermal capacity with an average degree of heating. The value for steel corresponds to 440 – 550 J/(kg deg), cast iron – 370 – 550 Joules/(kg deg), nickel – 440 J/(kg deg).
Special attention should be paid to the fact that the indicators of heavy metal components are not high. Therefore, famous physicists have identified the following relationship: the higher the density, the lower the heat coefficient.
Change in internal energy and temperature during solidification
During hardening, the opposite happens.
The average speed of movement of molecules and their average kinetic energy in a liquid (molten substance) decreases upon cooling . This corresponds to the section of the DE graph in Figure 2.
Now the attractive forces between the molecules can hold them close to each other. The arrangement of particles becomes ordered - a crystal is formed (section of the EF graph).
Where is the energy released during crystallization spent? The body temperature remains constant during this process. This means that energy is spent maintaining this temperature until the body completely hardens.
Now we can say that
At the melting point, the internal energy of a substance in the liquid state is greater than the internal energy of the same mass of substance in the solid state.
This excess energy is released during crystallization and maintains the body temperature at the same level throughout the solidification process.
Examples of problems
Given : $m = 2 \space kg$ $t_1 = 0 \degree C$ $t_2 = 100 \degree C$ $\lambda = 3.4 \cdot 10^5 \frac$ $с = 4.2 \cdot 10^3 \frac $
View solution and answer
Solution:
Then, to turn a piece of ice into boiling water, we need the amount of heat: $Q = Q_1 + Q_2 = 6.8 \cdot 10^5 \space J + 8.4 \cdot 10^5 \space J = 15.2 \cdot 10^5 \space J$ .
Given : $m = 10 \space kg$ $t_1 = 29 \degree C$ $t_2 = 1539 \degree C$ $c = 460 \frac$ $\lambda = 2.7 \cdot 10^5 \frac$
View solution and answer
Solution:
$Q_1 = cm(t_2 – t_1)$. $Q_1 = 460 \frac \cdot 10 \space kg \cdot (1539 \degree C – 19 \degree C) = 4600 \frac \cdot 1510 \degree C = 6 \space 946 \space 000 \space J \approx 69 \ cdot 10^5 \space J$.
$Q_2 = \lambda m$. $Q_2 = 2.7 \cdot 10^5 \frac \cdot 10 \space kg = 27 \cdot 10^5 \space J$.
$Q = Q_1 + Q_2 = 69 \cdot 10^5 \space J + 27 \cdot 10^5 \space J = 96 \cdot 10^5 \space J$.
Given : $m_1 = 3 \space kg$ $\lambda_1 = 3.4 \cdot 10^5 \frac$ $c_2 = 500 \frac$ $t_1 = 800 \degree C$ $t_2 = 0 \degree C$
View solution and answer
Solution:
Source