In order for a cutting tool to be used for cutting metal, it is necessary to apply appropriate force, provided that if the tool is strong enough, its hardness will be higher than the hardness of the material being processed. When cutting, the cutting part of the metal-cutting tool, which is in contact with the material being processed, is subjected to high pressure, heat and friction, which entails wear of the cutting tool, and sometimes its destruction. In this regard, the following requirements are imposed on the materials used for the manufacture of cutting tools:
- sufficient strength and hardness;
- wear resistance at high heating temperatures and over a long period of time.
The strength and hardness of the tool is determined by the hardness HRC (HRA) of the materials, the compressive strength σс, the bending strength σi, and the impact strength αн. Wear resistance is determined by the sufficient hardness of materials in a state heated to high temperatures - heat resistance (red resistance). Materials used for the manufacture of cutting tools :
- steels: carbon, alloy, high-speed, structural;
- hard alloys;
- diamonds;
- mineral ceramic materials;
- elbor;
- abrasive materials.
Tool carbon steels
The carbon content in steels is 0.6-1.4%, and the properties of the material largely depend on its value. The chemical composition and grades of tool carbon steels are given in GOST 1435-74 (replaced by GOST 1435-90, then GOST 1435-99). After appropriate heat treatment, carbon steels can have a hardness of HRC 58-64. However, when cutting, such a tool can withstand heating up to 200-250° C. At higher heating temperatures, the hardness of the tool sharply decreases (curve 8 in the figure), and it quickly collapses.
How is hardness determined?
Characteristics can be defined in different ways. Tool steels have Rockwell hardness, the hardness has a numerical designation, as well as a letter HR with a scale of A, B or C (for example, HRC). The choice of tool material depends on the type of metal being processed.
The most stable level of performance and low wear of blades that have undergone heat treatment can be achieved with an HRC value of 63 or 64. At a lower rate, the properties of tool materials are not so high, and at high hardness they begin to crumble due to brittleness.
Metals with a hardness of HRC 30-35 can be easily processed with iron tools that have undergone heat treatment with an HRC rating of 63-64. Thus, the ratio of hardness indicators is 1:2.
For processing metals with HRC 45-55, devices based on hard alloys should be used. Their indicator is HRA 87-93. Synthetic-based materials can be used when processing hardened steels.
Tool alloy steels
The cutting ability of tool carbon steel can be increased by adding alloying elements (additives), most often:
- chromium,
- molybdenum,
- tungsten,
- vanadium, etc.
Steels with such additives are called alloyed. After heat treatment, these steels are able to withstand heating to temperatures of 250-300°C during the cutting process. Tools made of alloy steels are capable of operating at speeds that are approximately 1.2-1.4 times higher than the cutting speed when working with tools made of tool carbon steels. The chemical composition of tool alloy steels, their grades and groups is determined by GOST 5950-73 (replaced by GOST 5950-2000). For the manufacture of cutting tools, alloy steels of the following grades are most often used: chrome-tungsten ХВ5, chrome-silicon 9ХС, chrome-tungsten manganese ХВГ. The change in hardness of tool and processed materials depending on temperature is shown in the figure.
Designations: 1 - TsM-332; 2 - VK2; 3 - T30K4; 4 - T15K6; 5 - VK8; 6 - T5K10; 7 – P18; 8 - U10; 9 - P9; 10 - 10ХНМА; 11 - 18HGT.
Topic 4.1. Materials for cutting and measuring tools.
Materials for cutting tools. Areas of rational use of various tool materials for blade and abrasive processing are given below, in Chapter 11 “Materials with Special Technological Properties”. It provides recommendations for the use of tool materials depending on the properties of the materials being processed, and in some cases, on the processing conditions.
Carbon and alloy tool steels. This group includes carbon steels and steels alloyed in relatively small quantities. The fundamental feature of such steels is that their high hardness is achieved only through martensitic transformation. Since the hardness of martensite is determined by its carbon content (see 5.4.2), in tool steels its content is high (0.7...1.3%).
Carbon and alloy tool steels do not have heat resistance. They retain high hardness when heated only to a temperature of about 200 °C. The main difference in the properties of steels in this group is hardenability, which depends on the level of alloying.
Carbon tool steels (seven grades from U7 to U13; recall that the number in the grade indicates the carbon content in tenths of a percent, for example in U12 steel 1.2% C) have low hardenability (for example, when quenched in water, U7 steel does not acquire through hardness even in a section of 12 mm, and the hardenability of U12 steel is less than 15 mm). Only small-sized instruments can be made from them. In addition, these steels are hardened by cooling in water. This determines the high probability of warping and deformation or even cracking. Quenching and cooling in water may also cause spotty hardness (see 5.5.2).
Alloyed tool steels. The purpose of alloying is to increase the hardenability of steels.
Low-alloy steels 11ХФ, 13Х and others have increased hardenability compared to carbon steels. Unlike carbon steels, low-alloy steels acquire high hardness 62...64 HRC in sections up to 20 mm after quenching in oil rather than in water.
Complex alloy steels KhVG, KhVSG, 9KhS have high hardenability. They are calcined (acquiring a martensite structure) in sections of 40...100 mm when quenched in oil. The advantage of alloy tool steels over carbon steels is that quenching in oil eliminates spotty hardness, the appearance of cracks, and reduces deformation (see 11.6).
Structure and heat treatment of steels. Almost all steels in this group are hypereutectoid (with the exception of U7 - hypoeutectoid and U8 - eutectoid). The carbide phase of these steels is cementite (M3C). In alloyed steels, some of the iron atoms in cementite can be replaced by atoms of alloying components. In steels alloyed with tungsten and vanadium, there is also a small amount of carbides based on tungsten (M6C) and vanadium (MC). The numbers in the formula show the number of metal (M) and carbon (C) atoms. These carbides have a complex composition. In addition to the atoms of the main carbide-forming component (iron in cementite, tungsten in M6C carbide), atoms of other components are present in these carbides (replace the main component) in certain quantities.
Annealing - Softening heat treatment of steels - is performed to improve machinability. The result should be a granular rather than lamellar perlite structure, which provides higher machinability. The annealing temperature is selected: for hypereutectoid steels – slightly higher than Ac1, hypoeutectoid steels – higher than Ac3. After isothermal holding at the specified temperatures, slow cooling (with a furnace) is carried out. As a result of annealing, steels acquire an equilibrium structure (in accordance with the “Fe – Fe3C” diagram, i.e. ferrite + pearlite for hypoeutectoid steels; pearlite - for eutectoid steels; pearlite + secondary cementite - for hypereutectoid steels).
The presence of a cementite network in the structure of hypereutectoid steels is unacceptable (plates of secondary cementite are located around pearlite grains, see 6.1.1). This leads to increased brittleness of the steel. To eliminate the defect, normalization is used - heating above Ast followed by cooling in air (see 5.5.4). In the annealed state, forming operations are performed, followed by hardening heat treatment.
Strengthening heat treatment of steels of this group consists of hardening and low tempering.
Hardening of hypoeutectoid steels is carried out from the temperature Ac3 + (30...50 °C), hypereutectoid steels - from the temperature Ac, + (50...70 °C). In order to reduce quenching stresses, step hardening can be used (see 5.5.2).
The structure of hardened steels is quenching martensite, retained austenite and secondary cementite (in hypereutskoid steels). In the event that the presence of retained austenite in the structure is unacceptable (for example, for a measuring instrument, since the decomposition of austenite during operation causes a change in size), cold treatment is performed after hardening. Steels of this group can be hardened with high-frequency heating, since their quenching temperatures are significantly lower than the melting onset temperatures, which is important, since high-frequency heating is carried out with significant overheating.
Temperature : 150...200 °C. At higher heating temperatures, noticeable softening occurs due to the decomposition of martensite and coagulation of cementite (see 5.5.3). During the process of low tempering, quenching stresses are reduced, which leads to a slight increase in strength and toughness, while the hardness decreases slightly (by 1...2 HRC) and remains high. The structure after tempering is tempered martensite, secondary cementite and retained austenite.
Properties and scope. After final heat treatment, the steel acquires a hardness of 62...65 HRC, a tensile strength of 2000...2500 MPa. Since steels of this group do not have heat resistance, their main area of application is tools operating at low cutting speeds (up to 5... 10 m/min). These are hand tools (taps, dies, reamers, files), as well as broaches, since broaching is carried out at low cutting speeds. Drills are also made from steels of this group.
When manufacturing broaches and dies, it is necessary to ensure minimal deformation during hardening. Broaches are characterized by a large ratio of length to diameter or thickness, which determines their low rigidity and thereby their susceptibility to warping during heat treatment. The cutting part of the dies, located in the middle of the tool, is not ground after heat treatment. For the manufacture of such tools, steels KhVG (broaches) and KhVSG (dies) are used due to their low tendency to deformation during heat treatment.
Files are made from U13 and 13X steels, and in mass production conditions, high-frequency hardening is used. Drills and taps are made of 9ХС steel.
Steels of this group are also used to make cold-formed tools and parts that require wear resistance provided by high hardness (equipment parts, machine guide bars, etc.).
High speed steels. Such steels are one of the main tool materials. Up to 60% of cutting tools are made from them. This is practically the entire range of shaped (complex shaped) cutting tools - cutters, cutters, shaver, etc.
The fundamental difference between high-speed steels and carbon and alloy tool steels is heat resistance. High-speed steels retain high hardness when heated to temperatures above 600 °C. High heat resistance of steels is achieved by eliminating the causes that cause softening of carbon and alloy tool steels when heated - decomposition of martensite and coagulation of carbides (see 9.2.1). The first condition can be ensured by a high level of doping of the solid solution, in which the diffusion of carbon is difficult, i.e. Martensite decomposition will occur at higher temperatures. In addition, the structure must contain a carbide phase that is highly resistant to coagulation when heated (i.e., not cementite). These conditions are met at high doping levels. Strong carbide-forming components are used as alloying components, forming their own carbides. High alloying of the solid solution is ensured by heat treatment, during which special carbides are dissolved in the solid solution. The main alloying components of high-speed steels are tungsten and (or) molybdenum, which are chemical analogues; The steel composition also necessarily includes chromium to increase hardenability and vanadium to preserve fine austenite grains during hardening. Depending on the presence of W and Mo, steels are divided into tungsten, tungsten-molybdenum and molybdenum.
The marking of high-speed steels is somewhat different from structural ones. They are designated by the Russian letter “P”, the number after which indicates the tungsten content in the steel. The chromium content in all high-speed steels is about 4% and is not indicated in the grade. Vanadium with a content of up to 2% and carbon with a content of 0.7...0.9% are also not indicated. The designations of these elements are included in the grade of high-speed steels only when their content is higher. Let us show the markings using the example of the most common high-speed steels. P18 steel contains 18% W, R6M5 steel – 6% W and 5% Mo; the content of carbon, chromium and vanadium in these steels is within the specified limits.
Structure, heat treatment and properties of high-speed steels
(The single composition of high-speed steels in the annealed state is ferrite, in which part of the chromium present in the steel and carbides of alloying components are dissolved; the structure is fine-grained (sorbite-like) pearlite. The main carbide is based on tungsten and molybdenum: M6C (1200 HV), in In the structure of steels R18 and R6M5 it is present in an amount of about 18%. In addition, the structure contains chromium carbides - M23C6 (hardness 1000 HV, amount about 9%) and vanadium - MS (2500 HV, amount 1.5...2%).
Carbides have a complex composition. In addition to the atoms of the main carbide-forming component (carbide base), they contain certain quantities of iron atoms and other alloying components. Thus, the composition of Me6C carbide may include atoms of chromium, vanadium, and iron.
By their nature, carbides are divided into eutectic and secondary. Let us recall that the position of points S and E on the “Fe – Fe3C” diagram in the presence of alloying components shifts towards lower carbon values. Therefore, even with a carbon content of about 0.7% (the minimum content for high-speed steels), the structure of cast high-speed steel, due to the high content of alloying components, contains eutectic - ledeburite. Thus, high-speed steels are ledeburite-class steels.
Secondary carbides are released from the solid phase - austenite due to a decrease in the solubility of carbon in it with decreasing temperature (similar to the release of cementite from austenite along the SE line in the “Fe - Fe3C” diagram), as well as as a result of pearlite transformation. The presence of eutectic in the structure reduces the mechanical properties, therefore, high-speed steel ingots are subjected to hot plastic deformation (forging, rolling) in order to improve the structure by crushing the eutectic. After this treatment, carried out in metallurgical plants, the steel is annealed. Annealing is also carried out in engineering plants after welding and forging to improve the structure and ensure satisfactory machinability. After these operations, steels may have coarse grains and increased hardness.
Annealing is a softening heat treatment, including heating above the α → γ transformation temperature (840...860 ° C for tungsten and 800...830 ° C for tungsten-molybdenum steels), long-term exposure at this temperature (at least 3 hours) and subsequent very slow cooling with speed no more than 25...30 °C/h. Annealing should provide a granular pearlite structure and a minimum hardness (not more than 255 HB) for satisfactory machinability. After plastic deformation and annealing, high-speed steel acquires the structure P + Kavt (large eutectic carbides) + K„ (small secondary ones). In the annealed state, forming operations are performed, after which hardening heat treatment is carried out.
Strengthening heat treatment of high-speed steels, including quenching and tempering, is carried out in order to obtain high values of hardness and heat resistance. During heat treatment, a complex mechanism of strengthening of steels of this class is realized - martensitic transformation followed by dispersion hardening. This ensures high alloying of martensite, obtained during hardening, and intensive dispersion hardening during tempering.
Hardening . Heating for hardening of high-speed steels is carried out to obtain a high alloying of the solid solution. This can be achieved by dissolving a large number of carbides in austenite. Chromium-based carbide Me23C6 completely dissolves in austenite at 1100 °C; the main carbide of high-speed steels Me6C dissolves intensively at temperatures above 1200 °C; Vanadium-based MeS dissolves at higher temperatures.
Thus, to ensure high alloying of the solid solution, the hardening temperature of high-speed steels must be above 1200 °C, i.e. exceed the dissolution temperature of the main carbide. Tungsten-based Me6C carbide dissolves in austenite at temperatures higher than molybdenum-based carbide, therefore the hardening temperature of tungsten steels is also higher than that of steels with molybdenum (1270...1290 °C for R18 and 1210...1230 °C for R6M5).
After quenching, some undissolved – excess – carbides remain in the structure. These are mainly carbides of eutectic origin, the dissolution of which is possible only in the liquid phase (melting of the tool is unacceptable), and some secondary carbides. The role of excess carbides is to inhibit grain growth (coarse grains reduce strength and toughness) during heating for hardening, which is forced to be performed at high temperatures to dissolve Me6C carbide in austenite.
A high concentration of carbon and alloying components in austenite leads to a decrease in the temperatures of the beginning (Mn) and end (Mk) of the martensitic transformation. Temperature Mk lies in the region of negative temperatures, therefore, a fairly large amount (up to 30%) of retained austenite is retained in the structure of hardened high-speed steels.
Thus, the structure after quenching is quenching martensite (M3), carbides (K) and retained austenite (Aost).
Vacation. When tempering high-speed steels, dispersion hardening and relief of quenching stresses must be implemented, i.e. transformation of quenched martensite (M3) into tempered martensite (Mo), as well as transformation of retained austenite into martensite (austenite does not have the required hardness). These problems are solved, firstly, by choosing the isothermal holding temperature during tempering and, secondly, due to the fact that tempering is performed multiple times.
Isothermal holding temperature during tempering is 550…570 °C. At this temperature, martensite is preserved and dispersion hardening occurs due to the release of a large amount of dispersed carbides based on alloying components from the solid solution. Tempering ensures maximum hardness. An increase in temperature above the optimum leads to coagulation of dispersed carbides, decomposition of martensite and, consequently, to a decrease in hardness. The hardness obtained as a result of tempering at high temperatures is called secondary, in contrast to the primary, quenching hardness.
Materials for control and measuring instruments. The measuring tool is designed to measure linear and angular dimensions, shape and surface characteristics of parts, as well as to determine hardness, i.e. properties of materials.
For the manufacture of measuring instruments, steels, hard alloys, and diamonds are used.
Steel has found the greatest use. They are used to make gauge lengths (measuring tiles), staples, templates, gauges, parts of calipers, micrometer inserts, hardness tester indenters - balls for Brinell and Rockwell instruments.
Steels for the manufacture of measuring instruments must have:
Ø – high hardness – 55…65 HRC, which provides good wear resistance;
Ø – dimensional stability over time;
Ø – ability to obtain high surface cleanliness during finishing operations.
Measuring tiles, gauges, parts of calipers and micrometers, and indenters of hardness testers are subjected to volumetric hardening. The measuring surfaces of the caliper jaws must have a hardness of at least 58 HRC, and the measuring surface of the depth gauge ruler must have a hardness of at least 46 HR C. The hardness of the tiles, gauges, heels and inserts of micrometers must be 59...65 HRC. To achieve such hardness, parts are made from steels with a high carbon content - carbon and alloyed tool steels, ball bearing steels:
Ø – calibers – made of steels U10A, U12A, X, ShKh15, ShKh15SG;
Ø – measuring tiles – made of steel X (1% C, 1.1% St), 12X1 (1.2% C, 1.5% Cr).
Hardening technology – hardening and low tempering. High hardness is achieved by obtaining martensite with a high carbon concentration; in addition, retained austenite is present in the structure. This structure is unstable. During operation, austenite may decompose, causing an increase in size (austenite has a maximum density due to the high atomic density of the fcc lattice, i.e., a minimum specific volume). In addition, changes in tool size over time can occur due to the decomposition of martensite (a decrease in its carbon content due to the release of cementite), which will lead to a decrease in size. Changes in tool dimensions are unacceptable (primarily this applies to plane-parallel length measures and gauges).
Stabilization of the structure and dimensions of tools made of tool steels is achieved by the following heat treatment technology:
Ø – hardening; in water – carbon steels, in oil – alloy steels;
Ø – cold treatment at -70°С to eliminate retained austenite;
Ø – low tempering of long duration – 120… …130 °C, 24…60 hours.
After grinding, before polishing, it is advisable to re-temper for 2…3 hours to eliminate grinding stresses, the relaxation of which during operation will cause changes in shape and (or) dimensions.
For tools of low rigidity, it is recommended to carry out a preliminary heat treatment of normalization and subsequent tempering at 600 °C for 1 hour. This treatment ensures a homogeneous structure, which reduces deformation during hardening.
Staples, templates, patterns made from sheet material, especially instruments with large dimensions, are subjected to surface hardening. Case-hardened (20, 20Х) and medium-carbon (50, 55) structural steels are used. Tools made from steels 20 and 20X are carburized, hardened in oil (steel 20X) or in water (steel 20). Tempering is carried out at 150...180 °C for 2...3 hours. Due to the insignificant thickness of the cemented layer, transformations in it have little effect on changes in the total volume of the tool, so tempering of a longer duration is not required.
Tools made from steels 50 and 55 are hardened with induction heating (High-frequency hardening). This allows for less deformation and allows for straightening. Tempering is carried out at 150...180 °C for 2...3 hours. The wear resistance of the hardened layer of steels 50 and 55 is less than that of the cemented layer.
For special tools that must be resistant to corrosion, corrosion-resistant chromium steel 40X13 is used. Heat treatment: hardening from 950...1000 °C with cooling in oil and tempering at 120...130 °C for 12 to 50 hours. Hardness - 55...57 HRC.
The working surfaces of the measuring instrument must be of very high quality. The standards regulate the parameters of surface roughness Ra (μm): for measuring tiles - no more than 0.07 (with a rougher surface, grinding of the tiles is difficult or impossible); for calibers - from 0.05 to 0.8 depending on the degree of accuracy.
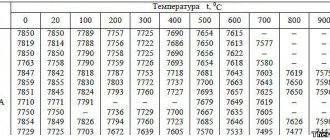
The best surface quality is achieved with a hardness of 63...65 HRC (the highest probability of obtaining the minimum Ra value, Table 9.15).
Table 9.15
What alloys have become widespread in industry?
Instrumental materials are produced all over the world. The types used in Russia, the USA and Europe, for the most part, do not contain tungsten. They belong to the KNT016 and TN020 series. These models became a replacement for the T15K6, T14K8 and VK8 brands. They are used for machining structural steels, stainless steel and tool materials.
New requirements for tool materials are caused by a shortage of tungsten and cobalt. It is precisely this factor that is responsible for the fact that alternative methods for producing new hard alloys that do not contain tungsten are constantly being developed in the USA, European countries and Russia.
For example, tool materials manufactured by the American company Adamas Carbide Co of the Titan 50, 60, 80, 100 series contain carbide, titanium and molybdenum. An increase in the number indicates the strength of the material. The characteristics of the tool materials of this release imply a high level of strength. For example, the Titan100 series has a strength of 1000 MPa. It is a competitor to ceramics.
Mineral ceramics
Mineral ceramics TsM-332 is used for cutting tools. It is characterized by resistance to elevated temperatures. The HRC hardness index ranges from 89 to 95 at 1200 °C. The material is also characterized by wear resistance, which makes it possible to process steel, cast iron and non-ferrous alloys at high cutting speeds.
To make cutting tools, B series cermet is also used. It is based on oxide and carbide. The introduction of metal carbide, as well as molybdenum and chromium into the composition of mineral ceramics helps to optimize the physical and mechanical properties of cermet and eliminates its fragility. Increases cutting speed. Semi-finishing and finishing machining with a cermet-based tool is used for gray ductile iron, difficult-to-cut steel and a number of non-ferrous metals. The process is carried out at a speed of 435-1000 m/min. Ceramics for cutting are resistant to temperature. Its hardness scale is HRC 90–95 at 950–1100 °C.
For processing hardened iron, durable cast iron, and fiberglass, a tool is used, the cutting part of which is made from solid substances containing boron nitride and diamonds. The hardness of CBN (boron nitride) is approximately the same as that of diamond. Its temperature resistance is twice as high as the latter. Elbor is inert to ferrous materials. The strength limit of its polycrystals during compression is 4-5 GPa (400-500 kgf/mm2), and during bending - 0.7 GPa (70 kgf/mm2). Temperature resistance reaches a limit of 1350–1450 °C.
Also worth noting is the synthetic-based diamond ballas of the ASB series and carbonado of the ASPC series. The chemical activity of the latter towards carbon-containing materials is higher. That is why it is used for sharpening parts made of non-ferrous metals, alloys with high silicon content, hard materials VK10, VK30, as well as non-metallic surfaces.
The resistance index of carbonate cutters is 20-50 times higher than the resistance level of hard alloys.
How can the performance of a cutting tool be increased?
Cutting tool materials provide increased functionality with increased temperature resistance and improved dissipation of heat generated at the blade during cutting. Heat causes the temperature to rise.
The more heat is transferred from the blade deeper into the device, the lower the temperature on its contact surface. The level of thermal conductivity depends on the composition and heating.
For example, the content of elements such as tungsten and vanadium in steel causes a decrease in the level of its thermal conductivity, and the admixture of titanium, cobalt and molybdenum causes its increase.