Iron is considered the most popular material. It is used in all industries. People have been familiar with this metal since ancient times. When blacksmiths learned to obtain pure material, it surpassed the alloys known at that time and forced them out of production. Iron alloys appeared as a result of people's attempts to change the characteristics of this metal.
Iron alloy
What is iron ore?
Technical iron does not exist under natural conditions, so it is always synthesized from ore. The percentage of iron depends on the location of the ore. As a rule, rock contains 15% iron.
Before iron was found in nature, people used bronze and copper, which are inferior to iron in reliability and strength. Therefore, people began to actively search for and use iron ore. Many centuries ago, craftsmen were able to distinguish the quality of ore without analyzing the elements.
History of discovery
Everyone remembers the “Iron Age” from school. This is the period in history when man first learned to extract this metal from ore. The Iron Age spans the period from the 9th to the 7th century BC. This metal had a huge influence on the development of people of that time. According to its characteristics, it has replaced mixtures of non-ferrous metals. Tools, weapons, armor, materials for construction and much more were made from it. Gradually, blacksmiths began to mix it with other metals to create new materials. This is how new alloys appeared.
What are iron-based alloys called?
An alloy is a mixture that includes not only the base metal, but also additional impurities. Iron-based compounds are called ferrous metals. These include a number of iron-based alloys.
- Steel is a combination of carbon with other chemical elements. The carbon content can be up to 2.14%.
- Cast iron is a mixture of carbon and manganese, phosphorus or sulfur. The carbon content can be up to 3.5%.
- Perlite is a compound of iron and carbon (up to 0.8%).
- Ferrite is a pure material with a carbon content of up to 0.04%.
- Austenite is a compound with a carbon content of up to 2.14%.
Industries that use iron media are the basis of civilization.
Composition and properties
The structure and properties of iron determine its popularity in various industries. The composition is a basic material with admixtures of other substances. The amount of additional metals does not exceed 0.8%. The main parameters include:
- Melting point - 1539 degrees Celsius.
- Brinell hardness - 350–450 MN/sq. m.
- Specific gravity - 55.8.
- Density - 7.409 g/cm3.
- Thermal conductivity - 74.04 W/(m K) (at room temperature).
- Electrical conductivity - 9.7·10-8 ohm·m.
We must not forget that iron is considered one of the most important elements in the human body. However, it is extremely difficult to absorb from food. The daily norm that a man should consume is 10 mg. Women should consume 20 mg of this substance for the body to function normally.
Methods for obtaining iron ore
Iron ore occurs at different depths, so different mining methods are used.
- Career way. The quarry method is relevant for the extraction of ore located at a depth of 200–300 m. Using excavators, ore is removed from the soil, and then the crushed raw materials are loaded and delivered to special plants.
- Mine method. The mine method is relevant for iron ore, which is located at a depth of up to 900 m. First, a mine alignment is pierced, from which drifts are developed along the seams. From the mines, crushed raw materials are transferred using conveyors. Then the raw materials are placed on machines and sent to special enterprises.
- Borehole hydraulic production. This method involves drilling a hole to the ore layer. Then pipes are passed into the target, which crush the ore with strong water pressure. This method has little effectiveness, so it is rarely used. About 3% of the ore is mined by borehole hydraulic mining.
Areas of application
This material is used in various industries:
- Mixtures and homogeneous metal are used in mechanical engineering. Internal parts, housings, and moving mechanisms are made from them.
- Shipbuilding, aircraft manufacturing, rocketry.
- Construction - production of fasteners, consumables.
- Instrument making - manufacturing electronics for the home.
- Radioelectronics - creation of elements for electrical appliances.
- Medicine, machine tool building, chemical industry.
- Making weapons.
If a homogeneous material is not suitable for something, compounds based on it, the characteristics of which differ significantly, will do.
Location of iron ore
The world's iron ore reserves are not unlimited. Raw materials for iron synthesis are not found on every corner. Territories in which large reserves of ores are located are called deposits. They are classified into types.
- Endogenous deposits are places with a special position in the Earth’s crust. They are often titanomagnetite ores. Outwardly, they resemble lenses and layers.
- Exogenous deposits – location of limonite, etc.
- Metamorphogenic deposits – location of quartzites, etc.
The countries of the former Soviet Union have huge reserves of iron ore. A large percentage of the rocks are found in Ukraine, the Russian Federation and Kazakhstan. Iron ore is also found in Brazil, Canada, Australia, the USA and other countries. But some countries have a shortage of iron in industries, so alloys often have to be imported.
State diagrams of two-component systems. Phases, structures
Alloys consist of two or more elements of the periodic table.
The elements that form alloys are called components. The properties of the alloy depend on many factors, but, first of all, they are determined by the composition of the phases and their quantitative ratio. Knowing the state diagram, you can imagine the full picture:
- formation of the structure of any alloy;
- determine the optimal temperature for pouring the alloy to produce cast parts;
- evaluate the fluidity of the selected alloy and the possibility of obtaining chemical heterogeneity;
- make a conclusion about the possibility and conditions of pressure treatment;
- determine the heat treatment regime required for a given alloy.
Basic definitions.
The phase diagram is a graphical representation of the phase composition of alloys of a given system as a function of temperature and chemical composition of the alloy (Fig. 1, Fig. 2)
Fig.1 Method for constructing state diagrams
Fig.2 Experimental setup for constructing phase diagrams
where: 1 – oven; 2 – crucible; 3 – molten metal; 4 – hot junction; 5 – thermocouple; 6 – cap; 7 – cold junction; 8 galvanometer
Experimentally constructed phase diagrams are checked according to the phase rule, which makes it possible to theoretically substantiate the direction of the transformation processes to establish the equilibrium state of the system.
The phase rule makes it possible:
- predict and test the processes occurring in alloys during heating and cooling;
- it shows whether the crystallization process occurs at a constant temperature or over a temperature range;
- indicates how many phases can exist simultaneously in the system.
The phase rule is expressed by the following equation:
C = K + 1 – F
where: K – number of components in the system; Ф – number of phases; C – number of degrees of freedom (or system variation). The number of degrees of freedom is the number of independent variables of internal (phase composition) and external (temperature, pressure) factors that can be changed without changing the number of phases in equilibrium.
State diagram of alloys, the components of which are completely soluble in liquid and solid states (Fig. 3).
Having a phase diagram, you can trace the phase transformations of any alloy and indicate the composition and quantitative ratio of the phases at any temperature.
This is done using two simple rules (Fig. 4):
- Concentration rule – rules for determining the composition of phases;
- The rule of segments is a rule for determining the quantification of phases.
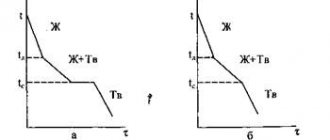
Fig. 3 Phase diagram with unlimited solubility and cooling curves for alloys and pure components
Fig.4 Application of the concentration rule and the segment rule
State diagram of alloys, the components of which are limitedly soluble in the solid state and form a eutectic (Fig. 5).
Fig.5 Construction of cooling curves for a diagram with limited solubility and with eutectic
State diagram of alloys with limited variable solubility of components in the solid state (Fig. 6).
Fig.6 Construction of cooling curves for a diagram with limited variable solubility
State diagram of alloys, the components of which are limitedly soluble in the solid state and form peritectics (Fig. 7)
Fig.7 Construction of cooling curves for diagrams with peritectics
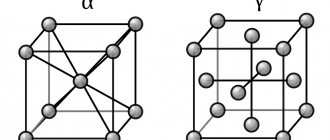
State diagrams of alloys, with polymorphic and eutectoid transformation of components (Fig. 8)
Eutectoid transformation : γE → αK + βF
Fig.8 Construction of cooling curves for a diagram with a polymorphic transformation
State diagrams of alloys, the components of which form chemical compounds (Fig. 9)
Fig. 9 State diagram, the components of which form chemical compounds
Physical and mechanical properties of alloys in an equilibrium state.
The properties of alloys are largely determined by the phase composition, which can be judged from the phase diagram (Fig. 10). The technological properties of solid solutions are of great practical interest.
Alloys in the state of solid solutions are well processed by pressure and difficult by cutting. The casting properties of solid solutions are, as a rule, unsatisfactory. Eutectic alloys have the best fluidity.
Intermediate phases in most cases have high hardness, melting point and brittleness (carbides, nitrides, borides, oxides, etc.).
The regularities noted by N.S. Kurnakov are the basis for the development of alloy compositions with specified properties.
Thus, in addition to classifying crystals by type of bond, classification by type of crystal lattice is used. This classification makes it possible to predict the nature of changes in the properties of the alloy as a function of composition.
Fig. 10 Relationship between the properties of alloys and the type of phase diagram (N.S. Kurnakov’s rule)
Use in construction
Steel and cast iron uniquely combine strength, durability and affordability. Therefore, it is not possible to replace it with any other structural material. In construction, rolled metal products are basic along with concrete and brick.
Capital construction
Metal can be given any shape: from the simplest - a rod, to the intricately complex - wrought iron. All options are used in construction.
In addition to the fact that steel itself is durable, especially after special treatment, another feature is actively used in this area. The fact is that profiled metal products are in no way inferior in strength to a solid part of the same size and shape . And this significantly reduces the material consumption of building elements, reduces their cost, reduces weight, and so on. In construction, this combination is extremely important.
The rolled metal used is divided into 3 main groups.
- Shaped - channels, I-beams, corner and regular profiles, as well as perforated. This also includes a special profile used, for example, in mine workings. Shaped metal products are used in the construction of all types of frames for any structure - from buildings to bridges and dams. It is also used when it is necessary to strengthen the structure.
- Sectional – fittings, beams, pipes, circles, etc. These elements are used almost more often than shaped elements and are very diverse: reinforcement - steel rods of different diameters, smooth and with ribs. The reinforcement is designed to increase the strength of the building, and the indicator is not only resistance to stationary loads, but also increased strength under tensile and bending loads. Reinforcement is used in the construction of foundations, floors, reinforced concrete slabs, strengthening walls, as well as in strengthening brickwork and other structural units - stairs, for example;
- pipes - both round and profile are used. Rectangular square pipes are preferable, since their welding and fastening is simpler than in the case of round ones, and the load resistance is the same;
- beam is an option for a solid cast product when strength is required under the highest loads.
- Rolled sheets – hot and cold rolled sheets with and without coating. These are roofing sheets, corrugated sheets, metal tiles and so on. Corrugated sheeting is used not only for roofing, but also in the construction of various fences, since the material combines relative lightness with high strength and resistance to temperature changes.
In construction, coated sheets are more often used - galvanized, for example, or with a polymer protective layer. This option is much more durable, as it is not subject to corrosion.
Stainless steels are rarely used for rolled sheets, since the cost of the alloy is higher.
Finishing work
They are often based on metal products - pipes, profiles, and sheet iron.
- Pipes of unusual shapes are actively used in modern interiors. They are used to construct sleeping blocks, floors and partitions in rooms, fences for both staircases and streets, and are even used in furniture production. Here, pipes, of course, are selected with a beautiful coating - nickel, chrome, although painted products are also available.
- Profile - niches and decorative projections, columns and ceilings, decoration of walls and fireplaces, etc., etc. Everything that is sheathed and covered with plasterboard, film, tiles, lining, panels - absolutely everything has a frame made of a metal profile. In the manufacture of furniture - sliding wardrobes, for example, a specialized profile is also used. Steel is much stronger and more durable than aluminum.
- Metal can act not only as a frame, but as a finishing material. Slat, cassette, and panel ceilings are extremely diverse, interesting and durable. Both slats and panels can be made of aluminum, but if a durable and strong solution is required - for example, for finishing the ceiling of a railway station, where resistance to vibration is required, steel is, of course, used.
- Doors are no longer classified as finishing work, but rather act as an element of the security system. Entrance doors made of steel of sufficient thickness are the most popular and reliable way to prevent home break-ins. The same can be said about garage doors, for example, or gates to the yard.
- Staircase structures - metal stairs are very diverse: from an attached or folding attic, to a permanent structure on the 2nd floor. This option is durable and reliable, and can also be very beautiful. Modern modular stairs are combined with glass, transparent plastic or even wood, and a stone staircase can be decorated with wrought iron railings.
Communications
Despite the fact that the steel pipeline is actively displacing plastic and metal-plastic pipelines, it is still extremely far from completely losing ground. The reason is simple: there is little that compares to the strength and durability of steel.
- Water supply and sewerage - if plastic products can be connected to service a private house or apartment, the same cannot be said about the main line or even the pipeline serving an apartment building. Only iron pipes are allowed, and they comply with firmly established standards.
- Gas pipeline - no options, only steel is used.
- Heating systems – in a building, the system may include plastic pipes. City and regional highways, not to mention the pipeline directly serving the boiler room, can only be made of iron. The initial temperature of the heated water is much higher than what plastic water lines can withstand, not to mention the pressure.
- As a rule, batteries and radiators are also made of iron or cast iron - cast iron has a higher heat capacity and resistance to water hammer. No matter what modern options the heaters are replaced with, steel is still present in the design. Electric radiators - convector, oil, are always made of steel, since the latter, having high thermal conductivity, instantly gives off heat to the air.
- Cables – wiring in the house is most often hidden in plastic boxes. However, power cables with a large cross-section are protected by metal pipes.
- Chimneys - steel pipes are the simplest, most affordable and easiest option. For their manufacture, special heat-resistant steel is used, which is resistant to corrosion.
Equipment and household items
Any equipment installed in the house is made of steel.
- Heating boilers - no matter what fuel the devices operate on, their bodies are always made of steel. Solid fuel stoves have cast iron parts.
- Kitchen equipment - stoves, ovens, microwaves, steamers and so on have steel bodies and parts. In the kitchen, steel is also a popular finishing material: worktops, for example, finishing an apron. Steel is a very decorative material and only seems simple.
- Washing machines, dryers and dishwashers also cannot do without iron.
- Steel plumbing fixtures are rarely used due to their high thermal conductivity, but cast iron bathtubs and washbasins are still installed. The material retains heat better and is very durable.
- Crockery and cutlery, coasters and vases, holders and fittings, electrical equipment and small accessories – you can count the places where iron is not used on your fingers.
- Wrought iron - decorative items of this kind are a real work of art, especially when it comes to hot forging, in which each product, each detail is made by hand and only once. Forged grilles, railings, fireplaces, fences decorate palaces and modern pavilions, and, of course, residential apartments.
Iron is the main structural material. In construction, steel and cast iron are basic materials along with building stone. The application and variety of alloys is beyond description.
Even more useful information on the use of iron is contained in this video: