Heat-resistant and heat-resistant steels and alloys.
HEAT-RESISTANT AND HEAT-RESISTANT STEEL AND ALLOYSSTRUCTURE AND MECHANICAL PROPERTIES AT ROOM AND HIGH TEMPERATURES (N.S. Samoilov)
Heat resistant
are called steels and alloys that retain high mechanical strength at elevated temperatures for a certain time and at the same time have sufficient heat resistance.
Heat-resistant (scale-resistant)
are called steels and alloys that are resistant to chemical destruction of the surface in gas environments at temperatures above 550 0 C, operating in an unloaded or lightly loaded state.
Heat resistance is characterized mainly by the limits of creep and long-term strength. Approximately, heat resistance is also judged by mechanical properties, determined by a short-term tensile test at operating temperature.
Additional characteristics of heat resistance: long-term ductility, relaxation resistance, endurance limit, heat resistance, etc.
The heat resistance of steel (alloy) is determined by the chemical composition and structure; elements that increase heat resistance include molybdenum, tungsten, vanadium, niobium, titanium, cobalt, aluminum and, to some extent, chromium and nickel. The latter, along with manganese, is important mainly as an austenite-forming element (since the austenitic structure creates the greatest heat resistance of steel). Chromium has less effect on heat-resistant properties than many other elements. However, its presence in steel or alloy along with aluminum and silicon increases their heat resistance (scale resistance). Therefore, chromium is an essential component of heat-resistant steels and alloys.
Classification
Heat-resistant steels include alloys based on iron if the iron content exceeds 50%.
Depending on the total content of alloying elements, heat-resistant steels can be low-, medium- and high-alloy.
In low-alloy steel, the total content of alloying elements does not exceed 4-5%. Medium alloy steel is a steel with a total content of alloying elements from 5 to 9%, and the content of each of them should not exceed 5%. High-alloy steel is called steel in which the content of any alloying element exceeds 5%, or the total content of all alloying elements is more than 10%.
Based on their microstructure (obtained after cooling in air at a high temperature), heat-resistant steels are divided into seven classes: pearlitic, martensitic, martensitic-ferritic, ferritic, austenitic-martensitic, austenitic-ferritic, austenitic.
Low-alloy steels belong to the pearlitic class, medium-alloy steels belong to the pearlitic, martensitic or martensitic-ferritic class, and high-alloy steels belong to any of the listed classes except pearlitic.
Iron-nickel alloys include alloys whose main structure is a solid solution of chromium and other alloying elements in an iron-nickel base. The total content of iron and nickel is not less than 65%.
Nickel-based alloys include alloys containing at least 50% Ni, the main structure of which is a solid solution of chromium and other alloying elements in nickel (iron content no more than 6-8%).
Pearlitic steels
Among low-alloy steels, molybdenum-containing steels are characterized by high heat resistance, for example, chromium-molybdenum, chromium-molybdenum-vanadium, chromium-molybdenum-tungsten-vanadium, which have fairly high creep resistance and long-term strength at temperatures up to 565-580 ° C. Such steels are conventionally called heat-resistant.
The chemical composition of heat-resistant pearlitic steels is given in GOST 20072-74, GOST 4543-71, TU 14-1-1391-75. They contain 0.5-3.3% Cr; 0.25-1.2% Mo; 0.15-0.8% V. Some brands contain 0.3-0.8% W or Nb.
These steels are used for the manufacture of various parts in the boiler industry, operating for a long time (10,000-100,000 hours) at temperatures of 500-580 ° C, in particular, for steam pipes and superheating pipes, as well as for rolled products and forgings used in turbines and steam high pressure boilers.
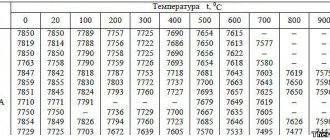
The mechanical properties of grade metal from pearlitic steels, provided for by GOST or existing specifications, as well as recommended heat treatment modes are given in Table. 1. Mechanical properties at elevated temperatures, determined by a short-term tensile test, are, as a rule, not regulated. Of decisive importance are the standards of long-term strength and creep at operating temperatures, depending on the service life over a period of 10,000-100,000 hours (Table 2). Information on the approximate purpose of pearlitic steels and their operating temperatures is given in table. 3.
Martensitic steels
Martensitic steels contain 4.5-12% Cr, as well as significantly smaller amounts of Ni, W, Mo, V.
Steel grades 15Х5, 15Х5М, 15Х5ВФ and 15Х8ВФ are widely used for the manufacture of equipment elements for oil refineries - parts of pumps, valves, fasteners, cracking pipes operating at temperatures of 550-600 ° C. Steels of the same group with a higher Cr content (6-10%) and a higher Si content (2-3%) are mainly used for the manufacture of valves for internal combustion engines.
Steel 11Х11Н2ВМФ (EI962) is used for compressor disks and other parts operating at temperatures up to 600 °C with a limited service life.
The mechanical characteristics of martensitic steels are given in table. 1 heat resistance characteristics - in table. 12.2.
Martensitic-ferritic steels
Steels of the martensitic-ferritic class contain 10-25% ferrite in their structure in addition to martensite. The main alloying additive in these steels is Cr (11-13%), along with which there are less significant additives Ni, W, Mo, Nb, V (modified chromium steels). Their heat treatment consists of either quenching and tempering, or normalizing and tempering. The mechanical properties at the proper tempering temperature are almost equivalent. The level of heat-resistant properties after optimal heat treatment for most steels of the martensitic-ferritic class is also approximately the same. However, the highest (when processed to the same hardness) heat resistance characteristics at 500-600 °C are for steel 18Kh12VMBFR (EI993).
These steels are produced in the form of rolled products and are used in turbine construction for turbine blades and disks, as well as for fasteners. The approximate operating temperature for steel 15Kh12VNMF(EI802) is 550-580 °C and 570-600 °C for steel 18Kh12VMBFR(EI993).
Austenitic steels
Austenitic steels are mainly chromium-nickel steels with a content of Cr and Ni ranging from 7 to 25% each, along with which there are W, Mo, Ti, Nb, etc.
This is the largest group of heat-resistant (and heat-resistant) steels (see GOST 5632-72).
Table 1
Heat treatment modes and characteristics of mechanical properties of long products made of heat-resistant steels at normal temperature
Steel
| Class | Heat treatment mode | Characteristics of mechanical properties | ||||||||
| Temperature of hardening or normalization, °C | Cooling medium | Temperature (or annealing), °C | Cooling medium | σв, MPa | σ0.2, MPa | δ5, % | ψ, % | KSU, J/cm2 | ||
| 12MH(12ХМ) | Pearlitic | 920 ± 10 | air | 680-690 | air | 420 | 260 | 21 | 45 | 60 |
| 15ХМ | 900-920 | air | 630-650 | — | 450 | 280 | 20 | 45 | 70 | |
| 12Х1МФ(12ХМФ,ЭИ575) | 960-980 | air | 740-760 | air | 480 | 260 | 21 | 55 | 100 | |
| 20ХМ | 860-880 | oil | 500-600 | air | 800 | 600 | 12 | 50 | 90 | |
| 25Х1МФ(ЭИ10) | 880-900 | oil | 640-660 | air | 900 | 750 | 14 | 50 | 60 | |
| 25Х2М1Ф(ЭИ723) | 1030-1060 | air | 680-720 | air | 900 | 750 | 10 | 40 | 30 | |
| 18Х3МВ(ЭИ578) | 960 ± 10 | oil | 660-680 | air | 650 | 450 | 18 | — | 120 | |
| 20Х3МВФ(ЭИ579) | 1030-1080 | oil | 660-700 | air | 900 | 750 | 12 | 40 | 80 | |
| 15Х5М | Martensitic | 950-980 | air | 860 ± 20 | air | 450 | 220 | 20 | 50 | 120 |
| 15Х5 | — | air | 850-870 | air | 400 | 170 | 24 | 50 | 100 | |
| 15Х5ВФ* | — | air | 850-870 | with oven | 400 | 220 | 22 | 50 | 120 | |
| 40Х9С2(4Х9С2,ЭСХ8)* | — | air | 850-870 | with oven | 750 | 500 | 15 | 35 | — | |
| 40Х10С2М(ЭИ107) | 1050 | air or oil | 750±30 | oil | 950 | 750 | 10 | 35 | > 20 | |
| 15Х11МФ | 1095 | oil | 710 | air | 755 | 568-755 | 14 | 50 | 59 | |
| 18Х11МNFБ(EP291) | 1080-1130 | air, oil | 660-770 | air | 740 | 590-735 | 15 | 50 | 59 | |
| 20Х12ВНМФ(EP428) | 1010-1060 | oil | 660-770 | air | 740 | 590-755 | 14 | 45 | 54 | |
| 30Х13Н7С2(ЭИ72) | 1050+800 | water, oil | 660-680 | air | 1200 | 800 | 18 | 25 | > 20 | |
| 11Х11Н2В2МФ | 1000-1020 | air or oil | 660-680 | air | 900 | 750 | 12 | 50 | 80 | |
| 16Х11Н2В2МФ(ЭИ962А) | 1000-1020 | Same | 550-590 | air | 1000 | 850 | 10 | 45 | 70 | |
| 20X13(EZh2) | 1000-1030 | Same | 680-720 | oil, air | 660 | 450 | 16 | 55 | 80 | |
| 13Х11Н2В2МФ-Ш(ЭИ961-Ш) | 1000-1020 | air, oil | 660-710 | air | 880 | 735 | 15 | 55 | 88 | |
| 12Х1 | Martensitic-ferritic | 1020-1050 | air or oil | 700-750 | oil | 600 | 420 | 20 | 60 | 90 |
| 15Х11МФ | 1030-1100 | air | 700-740 | oil | 700 | 500 | 15 | 55 | 120 | |
| 15Х12ВНМФ(ЭИ802) | 1000-1020 | air, oil | 540-590 | air | 1080 | 930 | 13 | 55 | 88 | |
| 15Х11ВНМФ | 1010-1060 | oil | 660-770 | air | 740 | 590-735 | 14 | 45 | 54 | |
| 18X12VMBFR(EI993) | 1050 | oil | 650-700 | air | 750 | 500 | 14 | 50 | 60 | |
| 18Х12ВМБФР-Ш(ЭИ993-Ш) | 1030-1050 | oil | 680-720 | air | 800 | 680 | 12 | 45 | 59 | |
| 15Х12В2МФ | 1050 | oil | 680 | air | 800 | 600 | 15 | 50 | 70 | |
| 20Х20Н14С2(DI911) | Austenitic-ferritic | 1000-1150 | air, water | — | — | 590 | 295 | 35 | 55 | — |
| 20Х23Н13(ЭИ319) | 1100-1150 | air, oil, water | — | — | 490 | 295 | 35 | 50 | — | |
* Steel is used in annealed condition
table 2
Heat treatment modes, creep limits and long-term strength of alloy steels of pearlitic and martensitic classes used for long service
| Steel | Class | Heat treatment mode | Test temperature, °C | Long-term strength limit, MPa per time, h | Creep limit, MPa, corresponding to 1% deformation over time, h | |||||
| Temperature of hardening or normalization, °C | Cooling medium | Temperature, °C | Cooling medium | 10 000 | 100 000 | 10 000 | 100 000 | |||
| 12MH(12ХМ) | Pearlitic | 920 | air | 680-690 | air | 480 | 250 | 200 | 220 | 150 |
| 510 | 160 | 120 | — | 700 | ||||||
| 540 | 110 | 70 | — | 38 | ||||||
| 12Х1МФ(12ХМФ,ЭИ575) | 960-980 | air | 740-760 | air | 520 | 200 | 160 | 180 | 130 | |
| 560 | 140 | 108 | 118 | 84 | ||||||
| 580 | 120 | 90-100 | 90 | 62 | ||||||
| 25Х1МФ(ЭИ10) | 880-900 | oil | 640-660 | water | 500 | 260-290 | — | — | 80 | |
| 550 | 100-150 | — | 90 | 30 | ||||||
| 25Х2М1Ф(ЭИ723) | 1050 | air | 680-700 | air | 550 | 180-220 | 140-480 | — | 70 | |
| 18Х3МВ(ЭИ578) | 900 ± 10 | oil | 660-680 | air | 450 | — | — | 230 | 160 | |
| 500 | — | — | 120 | — | ||||||
| 550 | — | — | 75 | — | ||||||
| 20Х3МВФ(ЭИ579) | 1030-1080 | oil | 660-700 | air | 500 | 340 | 300 | 180 | 150 | |
| 550 | 200 | 160 | 130 | 100 | ||||||
| 580 | 140 | 100 | — | 50 | ||||||
| 15Х5М | Martensitic and martensitic-ferritic, austenitic-ferritic | 950-980 | air | 860 ± 20 | air | 480 | 180 | 150 | 105 | 70 |
| 540 | 100 | 75 | 65 | 40 | ||||||
| 15Х5ВФ* | — | 860 ± 10 | 500 | 120 | 92 | 85 | 60 | |||
| 550 | 90 | 70 | 50 | 38 | ||||||
| 600 | 65 | 52 | 38 | 28 | ||||||
| 20Х12ВНМФ(EP428) | 1010-1060 | oil | 660-770 | air | 450 | — | — | — | 274 | |
| 500 | 382 | 343 | — | — | ||||||
| 600 | 103 | 88 | — | 54 | ||||||
| 12Х13 | 1030-1050 | oil | 730-750 | air | 470 | 260 | 220 | — | — | |
| 500 | 220 | 190 | — | 57 | ||||||
| 530 | 190 | 160 | — | — | ||||||
| 13Х11Н2В2МФ-Ш(ЭИ961-Ш) | 1000-1020 | air, oil | 660-710 | air | 500 | 392 | s100 = 568 | — | — | |
| 550 | — | s100 = 441 | — | — | ||||||
| 600 | — | s100 = 294 | — | — | ||||||
| 15Х12ВНМФ(ЭИ802) | 1000 | oil | 680 | air | 550 | 250 | 220 | — | 100 | |
| 565 | 240 | 200 | — | 80-90 | ||||||
| 580 | 190 | 160 | — | 70-80 | ||||||
| 600 | 140-160 | 120 | — | 50-60 | ||||||
| 15Х11МФ | 1050 | air | 740 | — | 550 | 200 | 130-150 | — | 90-100 | |
| 600 | 100 | — | — | 40-50 | ||||||
| 18X12VMBFR(EI993) | 1050 | oil | 650-700 | air | 560 | 250-300 | 220-260 | — | 150 | |
| 590 | 210-240 | 170-200 | — | 100 | ||||||
| 620 | 140 | 110 | — | 50 | ||||||
| 15Х12В2МФ | 1050 | oil | 680 | air | 575 | 170 | 150 | — | 75 | |
| 600 | 150 | 130 | — | 45 | ||||||
| 630 | 110 | 85 | — | — | ||||||
| 20Х20Н14С2(DI911) | 1000-1150 | air, water | — | — | 875 | — | — | 9,8 | — | |
| 1000 | — | — | 1,4 | — | ||||||
| 20Х23Н13(ЭИ319) | 1100-1150 | air, oil, water | — | — | 550 | 151 | 57 | — | — | |
- The steel is used in the annealed state
Table 3
Approximate purpose of low-alloy heat-resistant pearlitic steels
| Steel | Purpose | Operating temperature, ˚ C | Life time | Temperature of the beginning of intensive scale formation, ˚ C |
| 12MH(12ХМ) | Pipes of steam heaters, steam pipelines and manifolds of power plants; fittings for steam boilers and steam pipelines | 500-510 | Very long lasting | 570 |
| 15ХМ | 520-530 | 570 | ||
| 12Х1МФ(12ХМФ,ЭИ575) | 570-585 | 600 | ||
| 15Х1М1Ф | 570-585 | 600 | ||
| 18Х3МВ(ЭИ578) | Pipes for hydrogenation plants and petrochemical equipment | 450-500 | Long | 600 |
| 20Х3МВФ(ЭИ579) | 500-550 | 600 | ||
| 20Х3МВФ(ЭИ579) | Forgings (rotors, discs), bolts | 530-560 | 600 | |
| 25Х1МФ(ЭИ10) | Fasteners (bolts, studs), flat springs | 500-510 | Long | 600 |
| 25Х2М1Ф(ЭИ723) | 520-550 | 600 |
Table 4
Heat treatment modes and characteristics of the mechanical properties of long products made of heat-resistant austenitic steels (at normal temperature)
| Steel | Heat treatment mode | Characteristics of mechanical properties | ||||||
| Quenching temperature, °C. | Cooling medium | T , °C, duration of holiday or aging | Tensile strength σв, MPa | Yield strength σ0.2, MPa | Relative elongation δ5, % | Relative narrowing ψ, % | Impact strength KSU, J/cm2 | |
| 10Х11Н20Т3Р(ЭИ696) | 1150-1180 | air, oil | 750 (16 h) | 850 | 500 | 10 | 15 | 30 |
| 10Х11Н23Т3МР-ВД (ЭП33ВД) | 1170-1200 | air | 750 (16-25 h) | 900 | 600 | 8 | 10 | 30 |
| 37Х12Н8Г8МФБ-Ш (ЭИ-481Ш, 4Х12Н8Г8МФБ) | 1140-1160 | water | 670 (12-14 h) 770-800 (10-12 h) | 850 | 600 | 15 | 20 | — |
| 45Х14Н14В2М(ЭИ69) | ** | 820 | 720 | 320 | 20 | 35 | 50 | |
| 09Х14Н18В2Б | 1110-1140 | air | * | 500 | 200 | 35 | — | — |
| 09Х14Н19В2БР(ЭИ695) | 1100-1150 | air | * | 500 | 220 | 38 | 50 | 140 |
| 09X14N19V2BR1(EI695) | 1130-1160 | air | 750 | 520 | 220 | 30 | 44 | 120 |
| 37Х12Н8Г8МФБ-Ш (ЭИ-481Ш, 4Х12Н8Г8МФБ) | 1140 ± 10 | water | 770-800 | 850 | 600 | 15 | 20 | 25 |
| 30Х13Г18Ф | 1150 ± 10 | water | 700 (10 h) | 700 | 360 | 30 | 40 | 80 |
| 08Х16Н13М2Б(ЭИ680) | 1100-1150 | water, air | 750 | 560 | 220 | 40 | 50 | 120 |
| 10Х17Н13М2Т(ЭИ448) | 1050-1100 | water | * | 520 | 220 | 40 | 55 | — |
| 08Х17Н15М3Т(ЭИ580) | 1050-1100 | air | * | 500 | 200 | 35 | 45 | — |
| 08Х15Н24В4ТР(ЭП164) | 1130-1150 | air | 730-750 | 750 | 450 | 20 | 35 | 80 |
| 08Х15Н24В4ТР(ЭП164) | ** | air | 700 (16 h) | 700 | 400 | 15 | 30 | — |
| 12Х18Н9 | 1050-1100 | air, water | 700 (20 h) | 500 | 200 | 45 | 55 | — |
| 08Х18Н10Т(ЭИ914) | 1050-1100 | Same | 700 (20 h) | 520 | 200 | 40 | 55 | — |
| 12Х18Н9Т | 1050-1100 | Same | 700 (20 h) | 550 | 200 | 40 | 55 | — |
| 12Х18Н12Т | 1050-1100 | Same | 800 (10 h) | 550 | 200 | 40 | 55 | — |
| 08Х18Н12Б(ЭИ402) | 1050-1100 | Same | * | 500 | 180 | 40 | 55 | — |
| 36Х18Н25С2 | 1100-1150 | air, oil, water | * | 650 | 350 | 25 | 40 | — |
| 36Х18Н25С2 | 1200 | water | 800 (8 hours) | 855 | 550 | 17 | 18 | 50 |
| 30Х19Н9МВБТ | 1150-1180 | air, water | 750-800 | 680 | 350 | 35 | 40 | 60 |
| 31Х19Н9МВБТ(ЭИ572) | 1050 | water | 750 (15 h) | 680 | 350 | 25 | 25 | 70 |
| 55X20N4AG9M | 1160-1190 | water | 760-780 | 1000 | 650 | 8 | 10 | — |
| 20Х20Н14С2(DI911) | 1000-1100 | air, water | * | 600 | 300 | 35 | 30 | — |
| 20Х23Н13(ЭИ319) | 1050-1150 | Same | * | 500 | 300 | 35 | 50 | — |
| 20Х23Н18(EI-417) | 1100-1150 | Same | * | 500 | 200 | 35 | 50 | — |
| 20Х23Н18(EI-417) | 1030-1130 | water | * | 540 | 265 | 35 | — | — |
| 20Х25Н20С2(ЭИ283) | 1100-1150 | air, water | * | 600 | 300 | 35 | 50 | — |
*Available without vacation. **Without hardening
The following designations for alloying elements are accepted in the grades of these steels: A - N, B - Nb, B - W, G - Mn, K - Co, M - Mo, N - Ni, P - B, C - Si, T - Ti , F - V, X - Cr, Yu - Al. The number after the letter indicates the rounded (average) content of this element as a percentage (if the content is less than 1%, the number is not written). The exception is carbon, the content of which is expressed in tenths of a percent in the first two digits of the brand. For example, grade 45Х14Н14В2М(ЭИ69) has the following composition: 0.45% C, 14% Cr, 14% Ni, 2% W, and ≤ 1% Mo. The characteristics of the mechanical properties of long products made of heat-resistant austenitic steels, as well as optimal heat treatment modes, are given in Table. 4.
In accordance with the characteristics of alloyed austenite, the characteristics of the heat-resistant properties of austenitic steels are higher (Table 5) than those of heat-resistant steels of pearlitic or martensitic classes.
Steel 08Х18Н10Т(ЭИ914) is used as heat-resistant and heat-resistant. At temperatures up to 600 °C, steel has stable mechanical properties, it is resistant to intergranular corrosion and welds well. Steel of this grade is produced in the form of long products, forgings, sheets, pipes for power and chemical equipment. Steel 12Х18Н12Т has similar properties, which is used in the same fields of technology.
Chromium-nickel-tungsten austenitic steels (45Х14Н14В2М (ЭИ69)) have increased heat resistance and fatigue resistance at high temperatures. Steel 45Х14Н14В2М (ЭИ69) is used for exhaust valves of internal combustion engines. For long service life at temperatures of 600-650 °C, steel of the same type with a reduced C content (up to 0.15%) is recommended.
Austenitic steels are used, as a rule, for the manufacture of parts operating at temperatures of 650-700 °C for a very long time. The mechanical properties of these steels at a temperature of 20 °C are similar, but the limits of long-term strength and creep differ quite significantly (Tables 4, 5). The most heat-resistant of them are steel 09Х14Н19В2БР(ЭИ695)
and 09X14N19V2BR1(EI695)
, which are used for the manufacture of superheating and steam pipes for ultra-high pressure installations.
Chromium-manganese steels of grades 30Х13Г18Ф and 37Х12Н8Г8МФБ-Ш (EI-481Ш, 4Х12Н8Г8МФБ) are substitutes for heat-resistant steels with a higher nickel content. These steels have fairly high long-term strength at temperatures of 500-650 °C.
Table 5
Creep and long-term strength limits of heat-resistant austenitic steels used for long-term service*
| Steel | Temperature, °C | Long-term strength limit, MPa per time, h | Creep limit, MPa, corresponding to 1% deformation over time, h | ||
| 10 000 | 100 000 | 10 000 | 100 000 | ||
| 09Х14Н18В2Б | 600 | 180 | 140 | 120 | 110 |
| 650 | 140 | 110 | 105 | 85 | |
| 700 | 90 | 65 | 60 | 50 | |
| 09Х14Н19В2БР(ЭИ695) | 650 | 168 | 130 | 140 | 110 |
| 700 | 125 | 95 | 85 | 65 | |
| 750 | 70 | 55 | — | — | |
| 09X14N19V2BR1(EI695) | 600 | 260 | 230 | 250 | 170 |
| 650 | 215 | 190 | 200 | 140 | |
| 700 | 170 | 140 | 120 | 85-90 | |
| 12Х18Н10Т | 600 | 150 | 110 | — | 75 |
| 650 | 80-100 | — | — | 30-40 | |
| 30Х19Н9МВБТ | 600 | 240 | 220 | — | 110 |
| 650 | 170 | 150 | — | 80 | |
| 12Х18Н12Т | 600 | 170 | 135 | — | — |
| 650 | 105 | 75 | — | — | |
| 08Х16Н13М2Б(ЭИ680) | 600 | 200 | 150 | 140-170 | 90-120 |
| 650 | 130 | 60**-90 | 100-120 | 50-70 | |
| 700 | 60-70 | 30-50 | 60 | 20 | |
| 10Х17Н13М2Т(ЭИ448) | 550 | 280 | 240 | — | 110 |
| 600 | 180 | 130 | 110 | 60 | |
| 650 | 110 | 70 | 90 | 50 | |
| 700 | 40/80** | 30 | 55** | 28** | |
| 20Х20Н14С2(DI911) | 650 | — | — | 65 | — |
| 700 | — | — | 30 | — | |
| 800 | — | — | 10 | — | |
| 20Х23Н13(ЭИ319) | 550 | 240 | 200 | 150 | 60 |
| 600 | 190 | 150 | 70-80** | 50** | |
| 650 | 110 | 70 | 50-60** | 30** | |
| 700 | 60 | 36 | 30 | 14 | |
| 20Х23Н18(EI-417) | 600 | 150** | 100 | 90 | 60** |
| 650 | 110 | 60**-80 | 50-60 | 40**-54 | |
| 700 | 50**-60 | 35 | 35 | 28**-35 | |
| 800 | 21 | 12-21 | — | 7**-12 | |
| 20Х25Н20С2(ЭИ283) | Almost like steel 20Х23Н18 (EI-417) | ||||
* Heat treatment modes, see table. 4.
** Data from foreign sources for steels of similar chemical composition.
Iron-nickel alloys
Iron-nickel alloys can be divided into two groups: 1) containing 14-16% Cr and 32-38% Ni and 2) containing 20-25% Cr and 25-45% Ni (or Ni + Mn) . The alloys of the first group are additionally alloyed with tungsten and titanium and have high (approximately equal) heat resistance (Table 6). Alloys of the second group, due to the increased Cr content, are heat-resistant; in terms of heat-resistant properties, they are inferior to alloys of the first group, for example, alloy KhN38VT (EI703).
Alloys KhN35VT(EI612), KhN35VMT, KhN35VTYu(EI787) are supplied mainly in the form of hot-rolled and forged rods and strips, as well as forgings. Alloys KhN35V5T, KhN38VT (EI703) and 12Kh25N16G7AR (EI835) are mainly used to make hot-rolled and cold-rolled sheets and strips, and alloys KhN45Yu (EP747) are also used to make pipes. Basically, iron-nickel alloys are used for the manufacture of parts for steam and gas turbines.
Nickel-based alloys
Nickel-based alloys are divided into two groups (see GOST 5632-72): 1) alloys used primarily as heat-resistant, and 2) heat-resistant alloys that have the required minimum heat resistance (Table 7).
Table 6
Long-term strength and creep limits of iron-nickel based alloys *1
| Steel | Temperature, °C | Long-term strength limit, MPa for time, h | Creep limit*3, , MPa | ||||
| 100 | 500 | 1000 | 10 000*2 | 100 000*2 | |||
| KhN30VMT(EP437,VZh102) | 650 | 370 | — | 290 | 230 | 180 | 210 (1/104);14 (1/105) |
| 700 | 280 | — | 220 | 180 | 140 | ||
| 800 | 150-170 | — | 100-110 | 68 | — | ||
| ХН35ВТ(ЭИ612) | 600 | — | — | 320 | 270 | 230 | |
| 650 | — | — | 220-230 | 190-200 | 150-160 | 170 (1/104);130(1/105) | |
| 700 | — | — | 140 | 95 | 65 | 110 (1/104);80 (1/105) | |
| ХН35ВТУ(ЭИ787) | 600 | 650-680 | 550-580 | 520-550 | 420-450 | — | |
| 700 | 380-400 | 320-340 | 280-320 | 240-260 | — | ||
| 750 | 300-340 | 240-300 | 200-270 | 170-230 | — | 250 (0,2/100) | |
| 800 | 210-240 | 150-180 | 120-160 | — | — | 130 (0,2/100) | |
| ХН35В5Т | 650 | — | — | 280 | 200 | 160 | 180 (1/104);130 (1/105) |
| 700 | — | — | 200 | 150 | 120 | 120 (1/104);90 (1/105) | |
| 750 | 200 | — | 150 | 110 | 80 | 80 (1/104);60 (1/105) | |
| ХН38ВТ(ЭИ703) | 800 | 80-90 | — | 52 | — | — | 63 (5/100)*4 |
| 900 | 30-40 | — | — | — | — | 21 (5/100)*4 | |
| 1000 | — | — | — | — | — | 9 (5/100)*4 | |
| HN45Yu(EP747) | 1000 | 20 | — | — | — | — | |
| 1100 | 9 | — | 5 | — | — | ||
| 1200 | 5 | — | 2,5 | — | — | ||
*1 After optimal heat treatment.
*2 Extrapolated values.
*3 In brackets in the numerator - deformation in %, in the denominator - time in hours.
*4 Determined on conical samples.
Table 7
Limits of long-term strength and creep of nickel-based alloys*1
| Steel | Temperature, °C | Long-term strength limit, , MPa per time, h | Creep limits*3, , MPa | ||||
| 100 | 200 | 300 | 1000 | 10 000*2 | |||
| KhN65VMTYu(EI893) | 700 | > 600 | — | — | 400 | 300 | 300 (1/10 000) |
| 750 | 500 | — | — | 330 | 230 | 200(1/10 000) | |
| 800 | 300 | — | — | 200 | 140 | 120 (1/10 000) | |
| KHN70VMUT(EI765) | 600 | 780 | 750 | 740 | 650 | 530 | — |
| 700 | 450-500 | 420-470 | 400-450 | 310-350 | 220-240 | 200 (1/10 000) | |
| 800 | 220-250 | 210-230 | 190-220 | 140-160 | — | 80 (1/10 000) | |
| KhN70VMTYu(EI617) | 700 | 480-520 | — | 420 | 360 | — | 300 (0,2/100) |
| 800 | 280-300 | — | 210 | 180 | — | 170 (0,2/100) | |
| 850 | 180-200 | — | — | 100 | — | 170 (0,2/100) | |
| ХН80ТБУ(ЭИ407) | 650 | — | — | — | 400 | 300-260 | 350 (1/10 000) |
| 700 | — | — | — | 270 | 170-180 | 220 (1/10 000) | |
| ХН70МВТУБ(ЭИ598) | 700 | 480 | 420 | — | — | — | 180 (0,2/100) |
| 800 | 250 | 230 | — | — | — | — | |
| KhN67MVTYu(EP202) | 700 | 480-520 | — | 380-420 | 360-390 | 280-320 | 360 (1/1 000) |
| 800 | 280-300 | — | 230-250 | 180-200 | 120-150 | — | |
| 850 | 180-200 | — | 140-160 | 110-130 | 70-80 | — | |
| 900 | 120-140 | — | 90-100 | 70-80 | 40-45 | 60 (1/1 000) | |
| KhN75MBTYu(EI602) | 700 | 160-170 | 150 | — | — | — | — |
| 800 | 80 | 70 | — | — | — | 43 (5/100)*4 | |
| 900 | 29 | 22 | — | — | — | 14 (5/100)*4 | |
| ХН78Т(ЭИ435) | 700 | 105 | — | — | 32-35 | — | — |
| 800 | 45 | — | — | — | — | 18(5/100)*4 | |
| 900 | 15 | — | — | — | — | 7 (5/100)*4 | |
| KHN77TYUR(EI437B) | 600 | 680 | 660 | — | — | 450 | 720 (0,2/100) |
| 700 | 420 | 400 | — | 350 | 180 | 260 (0,2/100) | |
| 800 | 200 | — | — | 150 | — | 150 (0,2/100) | |
| ХН60У(ЭИ559А) | 800 | 60-80 | — | — | 40-50 | — | — |
| 900 | 35 | — | — | 20 | — | 24 (0,2/100) | |
| 1000 | 6 | — | — | — | — | 10 (0,2/100) | |
| ХН60ВТ(ЭИ868) | 800 | 110 | 95 | 87 | — | — | 83 (5/100)*4 |
| 900 | 52 | 43 | 40 | — | — | 34 (5/100)*4 | |
| ХН70У(ЭИ652) | 800 | 90-100 | — | 80 | — | — | — |
| 900 | 35-40 | — | — | — | — | 25 (5/100)*4 | |
| ХН75ВМУ(ЭИ827) | 850 | 270 (at least 50 hours); 250 (at least 65 hours) | |||||
*1 After optimal heat treatment.
*2 Extrapolated values.
*3 In brackets in the numerator - deformation in %, in the denominator - time in hours.
*4 Determined on conical samples.
The most commonly used alloys of the first group belong to the Ni-Cr-Ti-Al system. The presence of Ti and Al in these alloys in quantities exceeding their limiting solubility in solid solution at temperatures of 650-950 °C allows one to achieve a significant effect of dispersion hardening after quenching and tempering, due to the release of dispersed particles of the intermetallic phase such as Ni3(Ti, NiAl). This microstructure makes the alloy resistant to temperature effects at 700-800 °C and above.
The introduction of W and Mo (in total up to 10%), as well as Nb, into the dispersion-hardening alloys of this group additionally strengthens the solid solution, slows down the development of diffusion processes and increases the amount of dispersed strengthening phase. The amount of dispersed phase is also increased by increasing the total content of Ti and Al. All this leads to a significant increase in the heat resistance of alloys, which makes it possible to use them at temperatures up to 800-850 °C and high voltages.
Features of the composition of nickel heat-resistant alloys include the presence in them of small additives of surface-active elements (B, Ce, sometimes Ba and Mg), which contribute to the refining of the metal and the strengthening of grain boundaries, as well as a small content of impurities (S, P, Pb, etc.) .).
The heat treatment of these alloys consists of single or double heating to high temperatures (1080-1200 °C) with cooling, most often in air, and subsequent tempering at temperatures of 700-850 °C. For the greatest stabilization of the original structure, in relation to parts with a long service life, it is recommended to carry out multi-stage tempering at a gradually lowering temperature.
Heat-resistant nickel alloys are produced in the form of rolled products (round bars) and partly in the form of forgings of various configurations.
The main purpose of this group of high-alloy alloys is the manufacture of working blades and disks of gas turbines. Discs operate at higher stresses than blades (but at slightly lower temperatures), so the disc material must have high creep resistance (especially at the rim) and increased strength (at the hub).
The strength of nickel-based alloys remains high up to temperatures of 800-900 °C. Thus, at 800 °C, the tensile strength σв of the most alloyed alloys is 700-800 MPa, the 100-hour long-term strength is 250-300 MPa. At the same time, the plasticity characteristics δ and ψ are satisfactory at all test temperatures and decrease slightly in the temperature range of dispersion hardening (700-800 °C). The residual deformation of these alloys during long-term strength tests at 700-800 °C is about 3-10%.
In table Table 7 shows the heat resistance characteristics of nickel alloys.
For long service life, the best combination of long-term strength and ductility is found in the alloy KhN65VMTYu (EI893), which is widely used as a material for the blade apparatus of stationary gas turbines GT-6, GTN-9, GTK-10, GTK-16, GTT-12, GTA- 18, GTU-25, GTU-100. This alloy is the main blade material in stationary gas turbine construction. In addition, due to its exceptionally high relaxation resistance, this alloy is used for the manufacture of turbine fasteners.
Heat-resistant nickel alloys can be used to produce parts by casting (for example, precision investment casting).
The second group includes alloys of the grades KhN67MVTYu(EP202), KhN60Yu(EI559A), KhN70Yu(EI652), KhN78T(EI435), KhN60VT(EI868), KhN75MBTYu(EI602), used primarily as heat-resistant. These alloys, with the exception of the last two, are distinguished by a high Cr content (20-30%) and an almost homogeneous solid solution structure after the adopted heat treatment modes (heating to 1000-1200 °C with cooling in water or air). These alloys are produced in the form of cold-rolled or hot-rolled sheets mainly for parts of gas pipeline systems operating at moderate stresses under conditions of very high temperatures (up to 1100-1200 °C). In addition to sufficient manufacturability (rollability, stampability, weldability) and high resistance to gas corrosion (scale resistance), these parts must have good resistance to thermal fatigue (heat resistance). Nickel-based alloys meet all these requirements.
Heat-resistant sheet nickel alloys have increased ductility in cold and hot states, but the heat resistance is lower than that of alloys of the first group. Thus, long-term strength over 1000 hours is 40-60 MPa at 800 °C and 20-25 MPa at 900 °C (Table 7).
CREEP AND LONG-TERM STRENGTH LIMITS
At stresses below the yield point in metals, the phenomenon of creep is observed. Creep is continuous deformation under constant stress. At low loads and low temperatures it is reversible.
Creep becomes a problem at elevated temperatures (from about 0.4-0.6 Tm) and loads above a certain value (but less than the yield strength). Creep deformation is accompanied by changes in structure and, accordingly, mechanical properties. Unlike plastic deformation, which strengthens the metal, creep deformation leads to its softening. In addition to the constantly increasing deformation and increasing creep rate, cracks begin to appear in the metal and, over time, its destruction occurs.
The concept of heat resistance is associated with the phenomenon of creep. This is the ability to work under load with acceptable deformations and without destruction at elevated temperatures.
A quantitative characteristic of heat resistance is the creep limit (GOST 3248-60) and the long-term strength limit (GOST 10145-81).
long-term strength limit is the conditional maximum stress, under the influence of which a material at a given temperature is destroyed after a given period of time. This characteristic determines the ability of a material to resist destruction under prolonged exposure to temperature and load.
Creep strength and long-term strength decrease with increasing temperature and holding time. They should be considered as operating voltage limits at high temperatures.
FATIGUE STRENGTH
Cracks in metals originate and develop not only under static loads, but also under the influence of cyclic stresses. A fatigue crack originates in the surface layers (this is its distinctive feature) and slowly develops deeper with each cycle. Failure occurs when, due to a reduction in cross-section, the effective stresses exceed the destructive ones.
Accumulation of damage means that the more loading cycles, the less the load must be for the metal to “work” without collapsing. The process of gradual accumulation of damage in metal is called fatigue.
The second most important endurance is fatigue life. It determines the number of cycles that a metal can withstand at a given stress. Since fatigue cracks initiate at the surface, the condition of the surface is of particular importance for increasing durability under cyclic loading. Polishing, surface hardening, and absence of corrosion increase the endurance limit.
6. Metal alloys, their types and structure; solid solutions, chemical compounds, mechanical mixtures. Concept of state diagrams. Building a state diagram. Phase rule (Gibbs law)
A metal alloy is a material obtained by fusing two or more metals or metals with non-metals and having metallic properties. The substances that form an alloy are called components.
A phase is a homogeneous part of an alloy, characterized by a certain composition and structure and separated from other parts of the alloy by an interface. Types of alloys by structure. According to the nature of the interaction of the components, all alloys are divided into 3 main types: mechanical mixtures, chemical compounds and solid solutions. A mechanical mixture of two components A and B is formed if they are not capable of interaction or mutual dissolution. Each component crystallizes into its own crystal lattice. The structure of mechanical mixtures is heterogeneous, consisting of separate grains of component A and component B. The properties of mechanical mixtures depend on the quantitative ratio of the components: the more of a given component in the alloy, the closer the properties of the mixture are to its properties. A chemical compound is formed when alloy components A and B react chemically. Moreover, the ratio of the numbers of atoms in the compound corresponds to its chemical formula AmBn. A chemical compound has its own crystal lattice, which differs from the crystal lattice of its components. Chemical compounds have a homogeneous structure, consisting of grains of identical composition and properties.
Gibbs equation
The phase rule is written as follows:
where j is the number of phases (for example, aggregate states of matter);
v is the number of degrees of freedom, that is, independent parameters (temperature, pressure, concentration of components) that completely determine the state of the system at equilibrium and which can be changed without changing the number and nature of phases;
k is the number of components of the system - the number of individual substances included in the system minus the number of chemical equations connecting these substances. In other words, this is the minimum amount of substances from which each phase of the system can be prepared.
n is the number of variables characterizing the influence of external conditions on the equilibrium of the system.
At variable pressure and temperature, the phase rule reduces to the expression:
In the case of a one-component system, it is simplified to:
,
This shows, for example, that in a one-component system three phases (j=3) can coexist with the number of degrees of freedom v equal to zero, that is, at fixed pressure and temperature, which corresponds to a triple point on the phase diagram. Two phases (j=2) coexist with an arbitrary change in either pressure or temperature, when the second of these variables is not independent (v=1), that is, a line corresponds to two-phase equilibrium on the phase diagram. If there is one phase (j=1), the number of degrees of freedom of the system is two, that is, temperature and pressure can change independently within a certain region on the phase diagram - until the system is on one of the lines of two-phase equilibrium.
Sometimes the phase rule is written as follows:
that is, at equilibrium, the number of phases in the system is less than or equal to the number of components plus 2.
Mathematical interpretation of the phase rule[
Mathematics allows us to describe natural phenomena in symbolic language in various ways. A successful interpretation of the phase rule is possible using graph theory. The equation j + v = k + 2 can be viewed very clearly as the relationship between the vertices, edges, faces and volumes of a certain graph.
7. The main types of phase diagrams of binary alloys (types I-IV): mechanical mixtures, unlimited and limited solid solutions, chemical compounds. The rule of segments and the rule of leverage
Status diagram. The phase diagram shows the structure of the alloy depending on the ratio of components and temperature. It is constructed experimentally using the cooling curves of the alloys (Fig. 1). Unlike pure metals, alloys do not crystallize at a constant temperature, but within a temperature range. Therefore, there are two critical points on the cooling curves of alloys. At the upper critical point, called the liquidus
(tl), crystallization begins.
At the lower critical point, which is called the solidus
(tc), crystallization is completed.
The cooling curve of the mechanical mixture (Fig. 1, a) differs from the cooling curve of the solid solution (Fig. 1, b) by the presence of a horizontal section. In this area, crystallization of the eutectic occurs. A eutectic is a mechanical mixture of two phases that simultaneously crystallized from a liquid alloy. Eutectic has a certain chemical composition and is formed at a constant temperature.
Rice. 1 – Cooling curves of alloys: a – mechanical mixture, b – solid solution. The line of the phase diagram on which crystallization of the alloy begins upon cooling is called the liquidus line ,
and the line on which crystallization ends is
the solidus line .
Types of state diagrams. The state diagram of alloys forming mechanical mixtures (Fig. 2) is characterized by the absence of dissolution of the components in the solid state. Therefore, in this alloy, the formation of three phases is possible: liquid alloy L, crystals A and crystals B. The ACB line of the diagram is the liquidus line: in the AC section, upon cooling, crystallization of component A begins, and in the CO section, component B begins. Line DC is the solidus line, on it the crystallization of A or B is completed and at a constant temperature the crystallization of the eutectic E occurs. Alloys whose concentration corresponds to point C of the diagram are called eutectic,
their structure is a pure eutectic.
Alloys located on the diagram to the left of the eutectic are called hypoeutectic;
their structure consists of grains A and eutectic.
Those alloys that are located to the right of the eutectic in the diagram are called hypereutectic;
their structure consists of grains B surrounded by eutectic.
Rice. 2 – State diagram of alloys forming mechanical mixtures. The state diagram of alloys with unlimited solubility of components in the solid state is shown in Fig. 3. For this alloy, the formation of two phases is possible: liquid alloy and solid solution a. There are only two lines on the diagram, the top one is the liquidus line, and the bottom one is the solidus line.
| Rice. 3 – State diagram of alloys with unlimited solubility | Rice. 4 – State diagram of alloys with limited solubility of components in the solid state |
The state diagram of alloys with limited solubility of components in the solid state is shown in Fig. 4. Three phases can exist in this alloy - a liquid alloy, a solid solution of component B in component A and a solid solution of component A in component B. This diagram contains elements of the previous two. The ACB line is the liquidus line, the AOSEV line is the solidus line. Eutectic is also formed here; there are eutectic, hypoeutectic and hypereutectic alloys. Along the PO and EC lines, secondary crystals of aII and bII are released (due to a decrease in solubility with decreasing temperature). The process of separating secondary crystals from the solid phase is called secondary crystallization.
The state diagram of alloys forming a chemical compound (Fig. 5) is characterized by the presence of a vertical line corresponding to the ratio of components in the chemical compound AB. This line divides the diagram into two parts, which can be considered as independent diagrams of alloys formed by one of the components with a chemical compound. In Fig. Figure 5 shows a diagram for the case when each of the components forms a mechanical mixture with a chemical compound.
Rice. 5 - Diagram of the state of alloys forming a chemical compound
During crystallization, both the concentration of the phases changes (therefore, the composition of the liquid changes) and the amount of each phase (during crystallization, the amount of the solid phase increases and the liquid phase decreases). At any point
diagrams, when two phases exist simultaneously in an alloy, it is possible to determine the amount of both phases and their concentration.
For this purpose, the so-called lever rule , or the rule of segments ,
. 8. Relationship between the properties of alloys and the type of state diagram (Kurnakov diagram)
Since the appearance of the phase diagram, as well as the properties of the alloy, depends on what compounds or what phases formed the components of the alloy, there must be a certain connection between them. This dependence was established by N.S. Kurnakov.