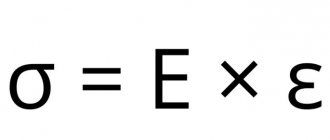History of discovery.
Also on topic:
CHEMICAL ELEMENTS
Zinc (like gold, silver, copper, mercury, lead, tin and iron) belongs to the metals of antiquity, the date of discovery of which is lost in the centuries.
The reduction of zinc oxide with charcoal requires a temperature of at least 1000 ° C. Since the metal at this temperature is in a vapor state and is easily oxidized, the isolation of zinc requires the ability to condense metal vapor, and this must be done in the absence of air, otherwise the metal will turn into oxide.
Also on topic:
CHEMICAL ELEMENTS IN NATURE – CYCLE AND MIGRATION
The production of zinc alloys from mixed ores does not require the isolation of zinc itself and is easier to achieve. The small amounts of zinc present in ancient Egyptian copper samples reflect the composition of local ores, but for the smelting of Palestinian brass dating from 1400–1000 BC. and containing about 23% zinc, copper ore must have been deliberately mixed with zinc ore. Brass was also obtained in Cyprus and, later, in the Cologne area (Germany). Chinese craftsmen mastered the art of zinc smelting in the Middle Ages. Zinc coins were used during the Ming Dynasty (1368–1644).
There was no dedicated production of zinc in medieval Europe, although small quantities were obtained in the production of lead, silver and brass. Beginning around 1605, it was imported from China by the East India Company. The English zinc industry emerged in the Bristol area in the early 18th century, and its products quickly spread to Silesia and Belgium.
The origin of the element's name is unclear, but it seems plausible that it is derived from Zinke (German for "point" or "tooth"), due to the appearance of the metal.
Distribution of zinc in nature and its industrial extraction. The zinc content in the earth's crust is 7.6 10–3%, it is approximately the same as rubidium (7.8 10–3%) and slightly more abundant than copper (6.8 10–3%) .
The main zinc minerals are zinc sulfide ZnS (known as zincblende or sphalerite) and zinc carbonate ZnCO3 (calamine in Europe, smithsonite in the US). This mineral received its name in honor of James Smithson, founder of the Smithsonian Institution in Washington. Less important minerals are hemimorphite Zn4Si2O7(OH)2·H2O and franklinite (Zn,Fe)O·Fe2O3.
Canada ranks first in the world in terms of production (16.5% of world production, 1113 thousand tons, 1995) and reserves of zinc. In addition, rich zinc deposits are concentrated in China (13.5%), Australia (13%), Peru (10%), USA (10%), Ireland (about 3%).
Zinc mining is carried out in 50 countries. In Russia, zinc is extracted from copper pyrite deposits in the Urals, as well as from polymetallic deposits in the mountains of Southern Siberia and Primorye. Large zinc reserves are concentrated in Rudny Altai (Eastern Kazakhstan), which accounts for more than 50% of zinc production in the CIS countries. Zinc is also mined in Azerbaijan, Uzbekistan (Almalyk deposit) and Tajikistan.
Characteristics of simple substances and industrial production of metallic zinc. Metallic zinc has a characteristic bluish luster on a fresh surface, which it quickly loses in humid air. Melting point 419.58° C, boiling point 906.2° C, density 7.133 g/cm3. At room temperature, zinc is brittle, at 100–150° C it becomes ductile and is easily rolled into thin sheets and wire, and at 200–250° C it again becomes very brittle and can be crushed into powder.
When heated, zinc reacts with non-metals (except hydrogen, carbon and nitrogen). Reacts actively with acids:
Zn + H2SO4(dil.) = ZnSO4 + H2
Zinc is the only element of the group that dissolves in aqueous solutions of alkalis to form [Zn(OH)4]2– ions (hydroxycinates):
Zn + 2OH– + 2H2O = [Zn(OH)4]2– + H2
When metallic zinc is dissolved in an ammonia solution, an ammonia complex is formed:
Zn + 4NH3 H2O = [Zn(NH3)4](OH)2 + 2H2O + H2
The initial raw materials for the production of metallic zinc are zinc sulfide and polymetallic ores. Isolation of zinc begins with the concentration of ore using sedimentation or flotation methods, then it is roasted to form oxides:
2ZnS + 3O2 = 2ZnO + SO2
The resulting sulfur dioxide is used in the production of sulfuric acid, and zinc oxide is processed electrolytically or smelted with coke.
In the first case, zinc is leached from the crude oxide with a dilute solution of sulfuric acid. In this case, cadmium is deposited with zinc dust:
Zn + Cd2+ = Zn2+ + Cd
The zinc sulfate solution is then subjected to electrolysis. Metal of 99.95% purity is deposited on aluminum cathodes.
The reduction of zinc oxide by coke is described by the equation:
2ZnO + C = 2Zn + CO2
Zinc smelting previously used rows of highly heated horizontal retorts intermittently, then these were replaced by continuously operating vertical retorts (in some cases, electrically heated). These processes were not as thermally efficient as the blast furnace process, in which the combustion of heating fuel is carried out in the same chamber as the oxide reduction, however the inevitable problem in the case of zinc is that the reduction of zinc oxide by carbon does not proceed below the boiling point of zinc ( This problem does not exist for iron, copper or lead), so subsequent cooling is necessary to condense the vapors. In addition, in the presence of combustion products, the metal is re-oxidized.
This problem can be solved by spraying the zinc vapors coming out of the furnace with molten lead. This results in rapid cooling and dissolution of the zinc, so that re-oxidation of the zinc is minimized. The nearly 99% pure zinc is then isolated as a liquid and further purified by vacuum distillation to 99.99% pure. All cadmium present is recovered during distillation. The advantage of the blast furnace is that the composition of the charge is not critical, so mixed ores of zinc and lead (ZnS and PbS are often found together) can be used to continuously produce both metals. Lead is released from the bottom of the furnace.
According to experts, in 2004 zinc production amounted to 9.9 million tons, and its consumption was about 10.2 million tons. Thus, the zinc deficit on the world market is 250–300 thousand tons.
In 2004, the production of refined zinc in China reached 2.46 million tons. Canada and Australia produce approximately 1 million tons each. The price of zinc at the end of 2004 was more than $1,100 per ton.
Demand for the metal remains high, thanks to rapid growth in the production of anti-corrosion coatings. To obtain such coatings, various methods are used: immersion in molten zinc (hot galvanizing), electrolytic deposition, liquid metal spraying, heating with zinc powder and the use of paints containing zinc powder. Galvanized sheet is widely used as roofing material. Metallic zinc in the form of bars is used to protect steel products in contact with sea water from corrosion. Zinc alloys - brass (copper plus 20–50% zinc) are of great practical importance. In addition to brass, a rapidly growing number of special zinc alloys are used for die casting. Another application is in the production of dry cell batteries, although this has declined significantly in recent years.
Approximately half of all zinc produced is used to produce galvanized steel, one third is used in hot-dip galvanizing of finished products, and the rest is used for strip and wire. Over the past 20 years, the global market for these products has more than doubled, increasing on average by 3.7% per year, and in Western countries, metal production is increasing annually by 4.8%. Currently, galvanizing 1 ton of steel sheet requires an average of 35 kg of zinc.
According to preliminary estimates, in 2005, zinc consumption in Russia may amount to about 168.5 thousand tons per year, including 90 thousand tons will be used for galvanizing, 24 thousand tons - for semi-finished products (brass, rolled zinc, etc.), 29 thousand tons - for the chemical industry (paints and varnishes, rubber products), 24.2 thousand tons - for foundry zinc alloys.
Physical properties
Several characteristics can be identified.
- Color: silver blue.
- Density - 7.13 g/cm3.
- Melting point - 420 °C (belongs to the group of low-melting metals).
- Under normal conditions it is a fragile substance.
- Boiling point - 906 oC.
- When heated to 100-150 °C, malleability and ductility increase, making wire and rolling into foil is possible.
- Above 200 °C it is easily ground into gray powder, losing its plasticity.
- He is an excellent guide.
- Thermal conductivity is good, as is the heat capacity.
Such physical parameters allow the metal to be used in connections with other elements. For example, a widely known zinc alloy is brass.
Zinc compounds.
Zinc forms numerous binary compounds with nonmetals, some of which have semiconducting properties.
Zinc salts are colorless (if they do not contain colored anions), their solutions have an acidic environment due to hydrolysis. Under the action of solutions of alkalis and ammonia (starting from pH ~ 5), the main salts precipitate and transform into hydroxide, which dissolves in an excess of the precipitant.
Zinc oxide
ZnO is the most important industrial zinc-containing compound. As a by-product of brass production, it became famous before the metal itself. Zinc oxide is obtained by burning zinc vapor in air, which is formed during the smelting of ore. A purer and whiter product is produced by burning vapors obtained from pre-purified zinc.
Typically, zinc oxide is a white, fine powder. When heated, its color changes to yellow as a result of the removal of oxygen from the crystal lattice and the formation of a non-stoichiometric phase Zn1+ x
O(
x
Ј 7.10–5). Excessive amounts of zinc atoms result in lattice defects that trap electrons, which are subsequently excited when visible light is absorbed. By adding a 0.02–0.03% excess of metallic zinc to zinc oxide, you can get a whole spectrum of colors - yellow, green, brown, red, but the reddish shades of the natural form of zinc oxide - zincite - appear for another reason: due to the presence manganese or iron. Zinc oxide ZnO is amphoteric; it dissolves in acids to form zinc salts and in alkalis to form hydroxinates, such as [Zn(OH)3]– and [Zn(OH)4]2–:
ZnO + 2OH– + H2O = [Zn(OH)4]2–
The main industrial application of zinc oxide is in rubber production, in which it reduces the vulcanization time of the original rubber.
As a pigment in the production of paints, zinc oxide has advantages over traditional lead white (basic lead carbonate), due to the lack of toxicity and darkening under the influence of sulfur compounds, but is inferior to titanium oxide in terms of refractive index and hiding power.
Zinc oxide increases the life of glass and is therefore used in the production of special glasses, enamels and glazes. Another important area of application is in neutralizing cosmetic pastes and pharmaceutical preparations.
In the chemical industry, zinc oxide is usually the starting material for the production of other zinc compounds, in which soaps (ie fatty acid compounds such as stearate, palmitate and other zinc salts) are the most important. They are used as paint hardeners, plastic stabilizers and fungicides.
A small but important application of zinc oxide is the production of zinc ferrites. These are spinels of the ZnII x
MII1–
x
FeIII2O4, containing another doubly charged cation (usually MnII or NiII). At x = 0 they have an inverted spinel structure. If x = 1, then the structure corresponds to normal spinel. A decrease in the number of FeIII ions in tetrahedral positions leads to a decrease in the Curie temperature. Thus, by changing the zinc content, it is possible to influence the magnetic properties of ferrites.
Zinc hydroxide
Zn(OH)2 forms as a gelatinous white precipitate when alkali is added to aqueous solutions of zinc salts. Zinc hydroxide, like the oxide, is amphoteric:
Zn(OH)2 + 2OH– = [Zn(OH)4]2–
Used for the synthesis of various zinc compounds.
Zinc sulfide
ZnS is released as a white precipitate when soluble sulfides and zinc salts react in an aqueous solution. In an acidic environment, zinc sulfide precipitate does not form in an acidic environment. Hydrogen sulfide water precipitates zinc sulfide only in the presence of weak acid anions, for example, acetate ions, which reduce the acidity of the medium, which leads to an increase in the concentration of sulfide ions in the solution.
Sphalerite ZnS is the most common zinc mineral and the main source of the metal, but a second natural, although much rarer, form of wurtzite is also known, which is more stable at high temperatures. The names of these minerals are used to refer to crystal structures, which are important structural types found for many other AB compounds. In both structures, the zinc atom is tetrahedrally coordinated by four sulfur atoms, and each sulfur atom is tetrahedrally coordinated by four zinc atoms. The structures differ significantly only in the type of close packing: in wurtzite it is cubic, and in sphalerite it is hexagonal.
Pure zinc sulfide is white and, like zinc oxide, is used as a pigment; for this purpose it is often prepared (as lithopone) together with barium sulfate by reacting aqueous solutions of zinc sulfate and barium sulfide.
Freshly precipitated zinc sulfide easily dissolves in mineral acids with the release of hydrogen sulfide:
ZnS + 2H3O+ = Zn2+ + H2S + 2H2O
However, calcination makes it less reactive and is therefore a suitable pigment in children's toy paints as it is harmless if swallowed. In addition, zinc sulfide has interesting optical properties. It turns gray when exposed to ultraviolet radiation (possibly due to dissociation). However, this process can be slowed down, for example, by adding traces of cobalt salts. Cathode, X-ray, and radioactive radiation produce fluorescence or luminescence of various colors, which can be enhanced by adding traces of various metals or by replacing zinc with cadmium and sulfur with selenium. It is widely used for the production of cathode ray tubes and radar screens.
Zinc selenide
ZnSe can be precipitated from solution as a lemon-yellow, difficult-to-filter precipitate. Wet zinc selenide is very sensitive to air. Dried or prepared dry, it is stable in air.
Zinc selenide single crystals are grown by directional crystallization of a melt under pressure or by vapor deposition. Zinc sulfide is used as a laser material and a component of phosphors (together with zinc sulfide).
Zinc telluride
ZnTe, depending on the production method, is a gray powder that turns red when rubbed, or red crystals, and is used as a material for photoresistors, infrared radiation receivers, dosimeters and radiation counters. In addition, it serves as a phosphor and semiconductor material, including in lasers.
Zinc chloride
ZnCl2 is one of the important zinc compounds in industry. It is obtained by the action of hydrochloric acid on secondary raw materials or roasted ore.
Concentrated aqueous solutions of zinc chloride dissolve starch, cellulose (which is why they cannot be filtered through paper) and silk. It is used in the production of textiles, in addition, it is used as an antiseptic for wood and in the manufacture of parchment.
Since zinc chloride easily dissolves oxides of other metals in the melt, it is used in a number of metallurgical fluxes. Using a solution of zinc chloride, metals are cleaned before soldering.
Zinc chloride is also used in magnesium cement for dental fillings, as a component of electrolytes for electroplating and in dry cells.
Zinc acetate
Zn(CH3COO)2 is highly soluble in water (28.5% by weight at 20° C) and many organic solvents. It is used as a fixative for dyeing fabrics, a wood preservative, an antifungal agent in medicine, and a catalyst in organic synthesis. Zinc acetate is a component of dental cements and is used in the production of glazes and porcelain.
When zinc acetate is distilled under reduced pressure, the basic acetate [Zn4O(OCOMe)6] is formed; its molecular structure includes an oxygen atom surrounded by a tetrahedron of zinc atoms connected along the edges by acetate bridges. It is isomorphic to basic beryllium acetate, but unlike it, it quickly hydrolyzes in water, this is due to the ability of the zinc cation to have a coordination number above four.
Organozinc compounds
. The discovery in 1849 by the English organic chemist Edward Frankland (1825–1899) of zinc alkyls, although not the first synthesized organometallic compounds (Zeise's salt was obtained in 1827), can be considered the beginning of organometallic chemistry. Frankland's research pioneered the use of organozinc compounds as intermediates in organic synthesis, and his measurements of vapor density led him to the proposition (crucial in the development of the theory of valency) that each element has a limited but definite strength of affinity. Grignard reagents, discovered in 1900, have greatly supplanted zinc alkyls in organic synthesis, but many of the reactions in which they are now used were first developed for zinc compounds.
Alkyls such as RZnX and ZnR2 (where X is halogen and R is alkyl) can be prepared by heating zinc in boiling RX under an inert atmosphere (carbon dioxide or nitrogen). Covalent ZnR2 are non-polar liquids or low-melting solids. They are always monomeric in solution and are characterized by linear coordination of the zinc atom
C–Zn–C. Organozinc compounds are very sensitive to air. Low molecular weight compounds spontaneously ignite, producing zinc oxide smoke. Their reactions with water, alcohols, ammonia and other substances proceed similar to Grignard reactions, but less vigorously. An important difference is that they do not react with carbon dioxide.
Chemical properties
Under normal conditions, the surface of a piece of metal is instantly covered with a dull gray-white coating - zinc oxide. This occurs because oxygen in the air instantly oxidizes the pure substance.
If pure metal is stored in open, humid air for a long time, it will be destroyed by carbon dioxide. How does the simple substance zinc react with:
- halogens;
- chalcogens;
- oxygen;
- acids;
- alkalis;
- ammonia and ammonium (its salts);
- weaker metals.
Does not react with nitrogen. If we take chemically absolutely pure zinc, then it does not react with any of the listed substances.
It is obvious that zinc is an amphoteric metal. When reacting with alkalis, it forms complex compounds - hydroxinates.
Biological role
What is zinc from a medical and biological point of view? Does it matter for the life of organisms and how great is it? It turns out that zinc ions simply must be present in living beings. Otherwise, the deficit will lead to the following consequences:
- anemia;
- decreased insulin;
- allergies;
- weight loss and memory;
- fatigue;
- depression;
- blurred vision;
- irritability and others.
The main places of concentration of zinc ions in the human body are the pancreas, liver and muscles. It is also this metal that is part of most enzymes (for example, carbonic anhydrase). Therefore, most catalytic reactions occur with the participation of zinc.
What exactly do ions do?
- Participate in the synthesis of male hormones and seminal fluid.
- Promotes the absorption of vitamin E.
- Participate in the breakdown of alcohol molecules in the body.
- They are direct participants in the synthesis of many hormones (insulin, growth hormone, testosterone and others).
- Takes part in hematopoiesis and healing of damaged tissues.
- Regulates the secretion of the sebaceous glands, maintains normal hair and nail growth, and promotes regeneration processes in the skin.
- It has the ability to eliminate toxins from the body and strengthen the immune system.
- Affects the formation of taste sensations, as well as the sense of smell.
- Takes part in transcription processes, vitamin A metabolism, nucleic synthesis and decay.
- It is a participant in all stages of cell growth and development, and also accompanies the process of gene expression.
All this once again proves how important this metal is. Its role in biological systems was clarified only in the 20th century. Many troubles and illnesses in the past could have been avoided if people had known about treatment with zinc-based drugs.
How can you maintain the required amount of this element in the body? The answer is obvious. It is necessary to consume foods containing zinc. The list can be long, so we will indicate only those with the maximum number of the element in question:
- nuts and seeds;
- legumes;
- meat;
- seafood, especially oysters;
- cereals and bread;
- milk products;
- greens, vegetables and fruits.
Being in nature
In total, scientists count about 66 zinc minerals or its derivatives. They learned to mix this metal with other elements, thereby obtaining various mixtures and different colors.
The primary mineral is considered to be the so-called zinc blende, which was discovered in Ancient Greece.
In nature, it occurs as a mixture with brown spar.
The earth's crust contains about 10% zinc. It can also be found in igneous earth rocks. The sour ones contain significantly less. Zinc hydroxide also occurs in nature. There are 5 modifications in total.
Zinc tends to migrate in thermal waters. In addition, it can be found in underground and surface waters, where it also migrates successfully.
Scientists have found that living organisms also contain relatively large amounts of zinc. They need it for successful life.