Properties of zinc
Zinc is in the second group of the periodic table. Like other elements in this group, it is divalent and has pronounced metallic properties. But as a metal, zinc is inferior in activity to beryllium, magnesium and alkaline earth metals, which represent the main subgroup of the same second group.
The serial number of zinc is 30, in the fourth row it is on the boundary between nickel and copper on the one hand and gallium, germanium and arsenic on the other. This transitional position between typical metals and non-metals explains the appearance of non-metallic properties in zinc, expressed in the amphotericity of its oxide.
The melting and boiling points of zinc are 427 and 907 ºС, respectively. The relatively low boiling point was the reason that delayed the development of zinc production. Conventional methods of smelting metals by heating a mixture of ore and coal did not produce results due to the volatility of zinc, which left the furnace space with flue gases in the form of vapors. Later they learned to condense vapors, which gave rise to the distillation method for producing zinc.
Natural zinc with an atomic mass of 65.37 consists of five isotopes: Zn64, Zn66, Zn67, Zn68, Zn70.
Electrochemical potential of zinc
Zn2+ + 2e = Zn; E0 = -0.76 V.
A large negative potential value characterizes high activity of zinc. However, it decomposes cold water; The reason for this is not only the thin film of basic carbon dioxide salts covering the metal, but also the slow discharge of hydrogen ions on zinc - a high hydrogen overvoltage on it.
Impurities of iron, copper and other more electropositive metals significantly accelerate the dissolution of zinc in acids.
To protect iron from corrosion, it is coated with a layer of zinc. With local destruction of the coating, the protection continues: exposed areas of iron are not destroyed, they become sites for the release of hydrogen due to the dissolution of zinc.
In strong acids, zinc dissolves with the release of hydrogen, and in alkali solutions - with the formation of zinc acid anions, zincates:
Zn + 2NaOH = Na2ZnO2 + H2.
Zinc oxide ZnO is a loose white powder obtained by burning zinc vapor in air; it is widely used for the production of oil and other paints: its pure white color and hiding power have made zinc white a necessary material in the painting industry.
Zinc oxide is an infusible substance: at temperatures above 1800 ºС it evaporates without melting. The temperature at which the reduction of zinc from carbon monoxide begins is about 950 ºС.
Zinc sulfide ZnS is also infusible and noticeably volatile at temperatures above 1180 ºC. When heated in air, ZnS is oxidized to basic sulfates - ZnO · nZnSO4, ZnSO4 sulfate and ZnO oxide.
Zinc in medicine
Biological role and functions of zinc in the body
According to scientific research, the adult body contains zinc in the amount of:
- for women - 1.5 grams
- for men - 2.5-3 grams
Percentage of zinc in the body:
- in the skin - 20%
- in the musculoskeletal system - 60%
The main function of the element for the human body is the formation of carbonic anhydrase in red blood cells to maintain acid-base balance. This protein ensures the conversion of carbon dioxide and its return to the lungs for subsequent exhalation. Zinc, being part of 400 digestive enzymes, affects the complete absorption of microelements from food.
In addition to its main properties, zinc performs the following functions:
- Affects the synthesis of hormones. Zinc influences: the activity of triple pituitary hormones; lipid and cholesterol metabolism; action of insulin in the blood.
- There is evidence of the participation of the metal in hematopoiesis and the formation of new cells. These are important functions during growth and for tissue regeneration.
- This microelement is especially important for men, as it actively promotes the production of testosterone. Zinc stimulates the production of seminal fluid and has a positive effect on prostate health.
- It is an antioxidant, neutralizes the effects of toxins and free radicals, preventing the development of cancer.
- Zinc controls the reproduction of DNA and RNA.
- Participates in collagen synthesis. This fibrillar protein forms the basis of connective tissue: joints, tendons, skin, bones, cartilage. Provides their strength and elasticity.
- Zinc “calms” the nervous system, improves mental activity, and has a positive effect on sleep.
- Zinc is the key to immunity. Participates in the metabolism of vitamins A, C, E, helps in the fight against allergies.
Foods rich in zinc
Zinc from food and vitamins is absorbed in the upper intestine through protein compounds from gastric juice. This microelement must be included in the diet of men, pregnant and lactating women and children during the period of their active growth.
TOP 10 food products by zinc content in mcg per 100 grams:
| 1. | Wheat germ | 17.000 |
| 2. | Sesame seeds | 7.800 |
| 3. | Oatmeal | 6.200 |
| 4. | Egg yolk | 5.600 |
| 5. | Beef liver | 5.000 |
| 6. | Peanut | 4.800 |
| 7. | Peas | 2.440 |
| 8. | Rabbit meat | 2.300 |
| 9. | Whole grain wheat bread | 2.130 |
| 10. | Buckwheat | 2.000 |
Daily intake of zinc
With proper nutrition, hypozincosis (zinc deficiency) is not dangerous for humans. Only if problems arise with the absorption of zinc salts from the intestine and its removal from the body, metabolic disorders may occur.
Zinc deficiency often occurs in children during active growth and in breastfed infants. Milk, unfortunately, contains a small amount of the element necessary for development.
In patients with diabetes, there is an increased excretion of zinc in the urine and, accordingly, lower levels of zinc in the body.
To increase the level of zinc in the body, it is recommended not only to add foods rich in this element to the diet, but also to take zinc-containing medications.
Recommended daily doses of zinc depending on gender and age:
- Newborns - 2 mg
- Babies from 6 months. up to 5 years - 3 mg
- Delhi up to 8 years - 5 mg
- Adolescents 8-14 years old - 8 mg
- Men - 15 mg
- Women - 10 mg
- Pregnant women - 15 mg
- Nursing mothers - up to 20 mg
Zinc is an essential nutrient with antioxidant and anti-inflammatory properties that is involved in a variety of biological processes in the human body.
Raw materials for zinc production
The main sources of zinc are sulfide, copper-lead-zinc, copper-zinc and lead-zinc ores.
In sulfide ores, zinc is usually present in the form of sphalerite or wurtzite, the composition of which corresponds to the formula ZnS, and marmatite nZnS · mFeS. Companions of zinc in polymetallic ores are minerals and elements.
In the oxidized zones of zinc-containing ore deposits, the main oxygen-containing zinc minerals are: smithsonite ZnCO3, zincite ZnO and calamine ZnO·SiO2·H2O. Oxidized zinc ores are currently of secondary importance.
In sulfide polymetallic ores, the zinc content is usually 1...3%. These ores have a complex composition. All this necessitates the preliminary enrichment of ores using a selective scheme to obtain several concentrates.
Zinc concentrates for selective flotation enrichment of polymetallic ores contain, %: Zn – 48...60; Pb – 1.5…2.5; Cu – 1…3; Cd – up to 0.25; Fe – 3…10; S – 30…38, waste rock – up to 10.
Zinc concentrates are complex, expensive raw materials. From them it is necessary to extract zinc, lead, copper, cadmium, sulfur, gold, silver, mercury, gallium, indium, thallium, selenium, tellurium, etc.
Sometimes, when beneficiating difficult-to-enrich copper-zinc ores, intermediate products containing 12...18% Zn and 4...8% Cu are obtained. Recycling of these materials is difficult in both zinc and copper plants.
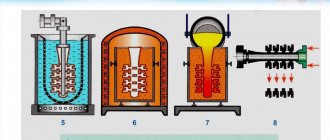
The processing of zinc concentrates is currently carried out by two methods - pyrometallurgical and hydrometallurgical.
The pyrometallurgical method is based on the process of reduction of zinc oxide at 1000...1100 ºС, i.e. at a temperature above the boiling point of metallic zinc, which ensures its release at the moment of formation in a vaporous state and sublimation in the form of vapors:
ZnO + C = Znsteam + CO; ZnO + CO = Znsteam + CO2.
The zinc vapor subsequently condenses. Obtaining liquid zinc by distillation is only possible under conditions of a highly reducing atmosphere and complete sealing of the equipment used.
Due to the fact that zinc concentrate is a sulfide material, and the recovery of zinc is possible only from its oxide, distillation is preceded by oxidative roasting with complete removal of sulfur.
There are several options for the hardware design of the pyrometallurgical method for producing zinc: in horizontal and vertical retorts, in shaft and electric furnaces. The principle of operation is the basis for the production of zinc vapor in the electrothermal part of the kivtset unit.
The zinc obtained by the pyrometallurgical method necessarily contains a large amount of impurity metals sublimated with it or entering it from dust carried by gases. Therefore, distillation zinc, like any rough metal, needs refining.
The pyrometallurgical method has been used since the emergence of zinc production. The share of zinc production by this method is decreasing from year to year and currently amounts to no more than 20%.
The hydrometallurgical method is currently the main one. The widespread use of hydrometallurgy in the production of zinc is due to its significant advantages over distillation. These include:
- extraction of more zinc and related elements;
- greater complexity of the use of raw materials;
- high quality zinc;
- high mechanization of labor-intensive processes.
Using this method, zinc is leached with a solution of sulfuric acid from a pre-calcined concentrate. During leaching, zinc goes into solution in the form of zinc sulfate by the reaction
ZnO + H2SO4 = ZnSO4 + H2O.
When zinc cinder is leached, the components contained in it partially pass into the solution. The quality of zinc obtained by electrolytic deposition depends on the purity of the solution: the purer the solution entering the electrolysis, the purer the commercial zinc obtained. Therefore, before electrolysis, the solution is thoroughly cleaned of impurities.
The process of electrolytic deposition of zinc from a purified solution proceeds according to the following overall reaction:
ZnSO4 + H2O = Zn + H2SO4 + 0.5O2
During electrolysis, zinc is deposited on the cathode, and sulfuric acid, necessary for leaching fresh portions of cinder, is regenerated at the anode, and oxygen is released. Zinc cathode deposits are melted down and poured into ingots.
Cake (undissolved sediment) obtained after leaching is subjected to additional processing in order to extract zinc and other valuable components from it.
Prevalence in nature and production
Zinc is a very common element. Natural zinc compounds include ZnS sulfide (two polymorphs: wurtzite and sphalerite ), also known as zinc blende; carbonate ZnCO3 - zinc spar.
Sphalerite, zinc blende
To obtain pure zinc, the ore is first roasted and then the resulting oxide is reduced with coal:
$$\ce{2ZnS + 3O_2 -> 2ZnO + 2SO_2}$$ $$\ce{ZnCO_3 -> ZnO + CO_2}$$ $$\ce{ZnO + C -> Zn + CO}$$
Methods for obtaining zinc
Two methods are used to extract zinc: pyrometallurgical (distillation) and hydrometallurgical (electrolyte).
Distillation method
Distillation in horizontal retorts
The zinc concentrate is fired to convert sulfides into oxides, sphalerite is oxidized by the reaction
2ZnS + 3O2 = 2ZnO + 2SO2.
A mixture of calcined zinc concentrate with fine anthracite or coke breeze is loaded into fireclay retorts installed horizontally in a furnace heated to 1400 °C.
In the retort, zinc is reduced by the reaction
ZnO + C = Zn(steam) + CO.
A condenser made of refractory clay is adjacent to the mouth of the retort; Liquid zinc is scooped out from it as it accumulates. However, not all zinc vapors have time to condense in the condenser; some of them go into the iron allonge, which is put on the mouth of the condenser. In the allonge, zinc is captured in the form of fine dust - poussières.
Other metals contained in the charge, such as cadmium, lead, and copper, can also be reduced in the retort. However, only cadmium and lead evaporate significantly and can contaminate zinc.
After distillation is completed, the condenser is removed, and the sintered residue from distillation, the rim, is unloaded from the retort. The rim contains 6–12% Zn; to extract it, the rim must be processed in a different way.
The pyrometallurgical method for producing zinc in horizontal retorts is essentially simple, but low-productivity and produces zinc contaminated with lead and cadmium.
The diameter of the horizontal retort cannot exceed 300–370 mm, and the thickness of its wall is 30–50 mm. As these dimensions increase, the heat transfer into the charge and the distillation rate deteriorate significantly. The length of the retort should not exceed 1700–1900 mm, otherwise at 1400 °C it will not withstand the bending load.
A retort of the indicated dimensions holds 80–90 kg of charge containing about 30 kg of zinc. With a distillation cycle lasting 24 hours and a liquid zinc yield of 80–83%, one retort produces no more than 25 kg of zinc per day. Therefore, in a modern plant of average capacity it is necessary to have several thousand retorts in operation. Retorts are still serviced manually - attempts to mechanize this work have failed.
Distillation in vertical retorts
It was possible to enlarge the retorts, and mechanize their maintenance only after the retorts were placed in a vertical position and made of carborundum.
Carborundum is silicon carbide, its chemical formula is SiC, its melting point is above 2700 °C. To make refractory products, powdered carborundum is mixed with 6–12% refractory clay. The mixture is moistened and pressed into molds, then dried and fired at 1400–1600 °C. The refractory products obtained in this way retain mechanical strength up to 2000 °C, they are chemically neutral and 3–4 times more thermally conductive than fireclay.
A vertical retort is a rectangular shaft assembled from carborundum slabs or lined with carborundum bricks.
The generator gas that heats the retort is burned in chambers on both sides of it. The height of the heated part is about 7.5 m. The charge is loaded from above in the form of briquettes, and a rim is continuously unloaded from the lower part of the retort, which essentially retains the original shape of the briquettes. To load the charge and remove zinc vapor, a chamber is made of refractory bricks above the retort. The lower part of the retort ends in an iron box with a water seal.
The charge is prepared from roasted zinc concentrate, anthracite, coking coal and a binder. After thorough mixing, the mixture is passed through a briquette press. Next, the briquettes are heated to 750–900 °C; At the same time, coal and resin coke, strengthening the briquettes and giving them the necessary porosity.
Distillation in vertical retorts is chemically no different from conventional distillation in horizontal retorts. The thermal conductivity of carborundum walls and briquetted charge is higher than during conventional distillation, therefore zinc is distilled off more completely, its content in the rimming is usually at least 3–5%.
The condenser is made of refractory brick; inside it has partitions that lengthen the path of gas movement.
The gases leaving the condenser are sent to a scrubber (a tall tower with a grid inside), where the remaining zinc is collected as a fine dust. The purified gases are burned in the combustion chamber of the retort; As a result, it is possible to save up to 20% of fuel.
The service life of the retort is 3–5 years, its productivity is 4–7 tons of zinc per day, or up to 90 kg per 1 m 2 of heat transfer wall per day.
A comparison of some indicators of zinc distillation in horizontal and vertical retorts is given in table. 9.
Distillation in electric furnaces
One of the disadvantages of vertical retorts is the need to transfer heat through the walls, which therefore have a higher temperature than the charge and wear out quickly. In this regard, the idea arose of heating the charge with an electric current passed through it, which led to the development of zinc electrothermy.
Zinc is produced using the electrothermal method in high shaft furnaces (12–14 m), made of high-grade refractory bricks. The charge consists of a cake of calcined concentrate and coke. The current is carried out by graphite electrodes installed at a distance of 8–10 m from one another in height, and flows through the coke. Numerous electric arcs arise between the pieces of coke, heating the mixture to an average of 1200 °C. The rim is unloaded continuously and contains 15–16% Zn. Zinc vapor is condensed into metal or burned to oxide, which is the final product. In addition to zinc, lead, copper and noble metals always remain in the rim, so it requires additional processing.
According to another method, distillation in electric furnaces is carried out when the charge is completely melted. The burnt concentrate is melted in a mixture with coal and fluxes at 1300–1350 °C, obtaining liquid slag, which serves as a heating body; graphite electrodes are immersed into it from above.
At a high temperature of the bath, even before the start of melting of the charge, not only oxides of copper and zinc, but also iron are reduced from it. Iron, dissolving carbon and copper, forms a layer of cuprous cast iron on the bottom. The total extraction of zinc into gases reaches 95%, but only 4/5 of it can be obtained in the form of metal, the rest turns into dust and oxides.
The energy consumption here is higher than during distillation from a solid charge; it reaches 3300 kWh per ton of zinc instead of 2550–2900 kWh using the first method. The advantages of distillation with melting of the charge are lower requirements for the quality of raw materials and greater complexity of its use.
In connection with the development of zinc electrothermy, capacitor designs were developed that make it possible to obtain the bulk of zinc in the form of metal even from rather poor gases. To do this, gases are sucked through a bath of molten zinc or liquid zinc is sprayed in a condenser with rotating mixers.
The large surface of the liquid metal promotes the condensation of vapors, even those significantly diluted with gases.