Decoding stamps
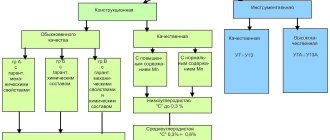
The marking of alloy steels consists of letters and numbers. At the beginning there is a two-digit number that characterizes the amount of carbon in hundredths of a percent. The following are letters of the Russian alphabet, denoting a specific element:
- X – chromium;
- N – nickel,
- T – titanium;
- B – tungsten;
- G – manganese;
- M – molybdenum;
- D – copper.
After the letter designation of the alloying element in the decoding there is a number indicating its content in stainless steel, rounded to the nearest whole percent. If there is no such figure, then the additive in the alloy is in the range of 1-1.5%.
General characteristics of corrosion-resistant steel
Corrosion-resistant steels include metal alloys that are highly resistant to corrosion processes in different atmospheric and climatic conditions, water, aggressive gas and chemical environments.
Anti-corrosion properties are ensured by enriching carbon steel with special elements, the most important of which is chromium. Its minimum content in the structure of alloys is 10.5%. Currently there are about 250 brands of stainless steel. The most used alloying elements are nickel, cobalt, titanium, molybdenum, niobium. Carbon, which is necessarily included in the composition, gives the finished products the necessary strength and hardness. Changing the proportions of chemical elements produces a metal with different properties, intended for specific areas of use.
Grades of heat-resistant and heat-resistant stainless steels
Heat resistance, otherwise called “scale resistance,” is the property of a metal to resist gas corrosion at high temperatures in an unloaded or lightly loaded state.
Definition! To improve this characteristic, chromium, silicon and aluminum are introduced into the composition of stainless steels. These elements, combining with oxygen, form dense structures that increase the resistance of steel to temperatures above +550°C. Nickel by itself does not affect heat resistance, but in combination with Cr, Al and Si it increases their efficiency.
Heat-resistant steels are steels that function at high temperatures and loads without a tendency to short-term and long-term creep.
Table of areas of application of scale-resistant and heat-resistant steels
| Type | Brand | Temperature at which active reaction with air begins, °C | Areas of use |
| Chromium, scale resistant | X18 | +850…+900 | Equipment, products and structures operated at T up to +900°C without load |
| High chromium, scale resistant | Х25 Х25Т Х28 | +1100…+1150 | Metal products intended for operation without load up to T +1150°C, Х25Т – for the production of thermocouples |
| Silchrome, scale-resistant | Х25С3Н | +1100 | For heating units and heaters operating at temperatures up to +1100°C |
| Highly alloyed, scale resistant and heat resistant | Х23Н18 | Loaded products and structures intended for operation at T up to +1000°C | |
| Х20Н35 | Metal products operated at T +1000°C |
Application
The listed advantages contribute to maintaining a leading position in the rolled metal market. Anti-corrosion alloys are an indispensable material in heavy engineering, energy, oil and gas and agricultural sectors.
The material is in demand in the following areas of the national economy:
- Construction, architecture;
- production of equipment and medical instruments;
- pulp and paper production;
- food industry;
- transport engineering;
- chemical industry;
- electrical power and electronics;
- production of household appliances and household items.
The decorative qualities of stainless metals and the high level of anti-corrosion properties make it possible to use parts and elements made from them for facades, advertising installations, shop windows, and fountains. Railings, doors, stairs, and elevators are made from alloyed material.
Application of heat-resistant steels
Alloy metals, resistant to high thermal loads, are used for the production of pipes, parts, components of machines, units, and industrial equipment. This list includes:
- parts of thermal furnaces;
- parts of conveyor belts for furnace conveyors;
- heat treatment units;
- fuel combustion chambers;
- motors, gas turbines;
- methane conversion devices;
- furnace screens;
- exhaust systems; heating elements.
Heat-resistant stainless metal is the best material for the production of parts and mechanisms that will be used in aggressive environments at elevated temperatures.
Sources
- https://met-all.org/stal/marki-nerzhaveyushhej-stali.html
- https://generalsteel.ru/marki-nerzhaveyushchih-stalej/
- https://vt-metall.ru/articles/447-svojstva-nerzhaveyushhej-stali
- https://martensit.ru/stal/nerzhaveyushhaya-stal/
- https://tk-metal.ru/stal/marki-nerzhaveyushchei-stali-klassifikatsiya-rasshifrovka.html
- https://solidiron.ru/steel/marki-nerzhaveyushhejj-stali-i-ikh-kharakteristiki.html
- https://intehstroy-spb.ru/spravochnik/nerzhaveyuschaya-stal-marki-vidy-i-harakteristiki.html
- https://prompriem.ru/stal/pishhevaya-stal-marki.html
Stainless steel grades for the manufacture of chimneys
When purchasing modular chimney systems, you need to find out what kind of steel they are made of. On sale you can find chimneys that are about one and a half times cheaper than other products in this category. In their production, AISI 201 steel (12X15G9ND) is used. According to international standards, it is necessary to use steel grade AISI 321 (08Х18Н12Т), the cost of which is approximately 2 times higher than the cost of AISI 201. It is impossible to visually distinguish AISI 201 from AISI 321, moreover, both alloys are non-magnetic. They can only be distinguished by chemical analysis.
Differences in chemical composition
| Brand | WITH | Mn | P | S | Si | Cr | Ni | Cu | Ti |
| AISI 201 | Up to 0.15% | 7-9,5 | Up to 0.1% | Up to 0.03% | Up to 1.0% | 13-18 | 0,3-3,0 | 0,5-2,5 | — |
| AISI 321 | Up to 0.08% | Up to 2.0 | Up to 0.05% | Up to 0.03% | Up to 1.0% | 17-19 | 9,0-12,0 | — | Min 0.5% |
AISI 201 steel has low anti-corrosion characteristics, instability of the structure, and the risk of cracks during drawing. Its use will lead to rapid failure of the chimney due to rapidly developing corrosion. This steel is mainly distributed in China and India.
Well-known foreign and conscientious Russian manufacturers, in addition to AISI 321 steel, use high-alloy alloys stabilized by Ti. They are acid and heat resistant. The use of cheaper steels (AISI 409, AISI 430) for gas exhaust pipes that do not meet acid resistance requirements leads to their failure soon after the start of the heating season.
Classification
Based on their chemical composition, stainless steels are divided into:
There are austenitic stainless steels, which are prone to intergranular corrosion, and stabilized ones - with Ti and Nb additives. A significant reduction in the susceptibility of stainless steel to intergranular corrosion is achieved by reducing the carbon content (up to 0.03%).
Stainless steels that are prone to intergranular corrosion are usually subjected to heat treatment after welding.
Alloys of iron and nickel are widely used, in which, due to nickel, the austenitic structure of iron is stabilized, and the alloy turns into a weakly magnetic material.
Martensitic and martensitic-ferritic steels
Martensitic and martensitic-ferritic steels have good corrosion resistance in atmospheric conditions, in slightly aggressive environments (in weak solutions of salts, acids) and have high mechanical properties. They are mainly used for products subject to wear, as cutting tools, in particular knives, for elastic elements and structures in the food and chemical industries that are in contact with slightly aggressive environments. This type includes steel types 30Х13, 40Х13, etc.
Ferritic steels
These steels are used for the manufacture of products operating in oxidizing environments (for example, in nitric acid solutions), for household appliances, in the food industry, light industry and for heat exchange equipment in power engineering.
Ferritic chromium steels have high corrosion resistance in nitric acid, aqueous solutions of ammonia, ammonium nitrate, a mixture of nitric, phosphoric and hydrofluoric acids, as well as in other aggressive environments. This type includes steels of the 400 series.
Austenitic steels
The main advantage of austenitic steels is their high performance characteristics (strength, ductility, corrosion resistance in most working environments) and good manufacturability. Therefore, austenitic corrosion-resistant steels have found wide application as a structural material in various branches of mechanical engineering. Theoretically, products made from austenitic stainless steels are non-magnetic under normal conditions, but after cold deformation (any mechanical treatment) they can exhibit some magnetic properties (part of the austenite turns into ferrite).
Austenitic-ferritic and austenitic-martensitic steels
Austenitic-ferritic steels
The advantage of steels in this group is an increased yield strength compared to austenitic single-phase steels, a lack of tendency for grain growth while maintaining a two-phase structure, a lower content of highly deficient nickel and good weldability.
Austenitic-ferritic steels are widely used in various branches of modern technology, especially in chemical engineering, shipbuilding, and aviation. This type includes steel types 08Х22Н6Т, 08Х21Н6М2Т, 08Х18Г8Н2Т.
Austenitic-martensitic steels
The needs of modern technology for corrosion-resistant steels with increased strength and manufacturability have led to the development of martensitic (transitional) class steels. These are steel types 07Х16Н6, 09Х15Н9У, 08Х17Н5М3.
Iron-nickel and nickel-based alloys
In the manufacture of chemical equipment, especially for work in sulfuric and hydrochloric acids, it is necessary to use alloys with higher corrosion resistance than austenitic steels. For these purposes, alloys based on iron-nickel base type 04ХН40МДТУ and alloys based on nickel-molybdenum base N70MF, chromium-nickel base KHN58V and chromium-nickel-molybdenum base KHN65MV, KHN60MB are used.
Stainless steels for the food industry
Corrosion-resistant steels are indispensable for industries producing equipment, tools and utensils intended for contact with food products. Their advantages:
- Resistance to various types of corrosion - chemical and electrochemical. In each specific case, it is necessary to select brands that are resistant to the environments with which they will come into contact during operation. These are normal atmospheric conditions, water, salt water, acidic, alkaline, chloride solutions.
- Good machinability. Modern tools make it possible to weld, cut, form and process corrosion-resistant alloys on lathes, milling and drilling machines in the same way as “ferrous” steels.
- Compliance with sanitary and hygienic standards. Thanks to various processing methods - grinding, polishing to a mirror finish - a surface is obtained that is practically free of pores and cracks into which dirt and pathogenic microorganisms can penetrate.
- Good mechanical characteristics. Thanks to them, it is possible to produce products and structures of smaller thickness and weight without compromising technical properties. Austenitic steels are more resistant to low temperatures compared to general purpose metals.
- Aesthetics. Electropolishing, satin finishing and other surface treatment methods provide a stylish look to stainless steel products.
Read also: Connector for Internet cable
Table of properties and areas of application of food grade stainless steel
| Steel grade according to GOST | AISI | Characteristics | Areas of use |
| 304 | 08Х18Н10 | It welds well, can be electropolished, retains high strength at normal and low temperatures, and is resistant to intercrystalline corrosion. | Equipment, tools, technological pipelines of food, petrochemical, pharmaceutical and medical industries, for utensils intended for high-temperature processing of products are not used |
| 316 | 03Х17Н14М2 | The presence of molybdenum increases the technical characteristics of the alloy at high temperatures | Installations, technological equipment, containers for the food and chemical industries |
| 321 | 12Х18Н10Т | Good weldability, maintaining performance characteristics at temperatures up to +800°C | Equipment for the chemical and oil refining industry |
| 409 | 08Х13 | Characteristics are satisfactory | Crockery and cutlery |
| 410 | 12Х13 | Heat resistance, resistance only to mildly aggressive environments | Winemaking equipment, alcohol containers |
| 420 | 20Х13-40Х13 | Versatility, ductility, wear resistance, increased corrosion resistance | Dishes, kitchen sinks |
| 430 | 08Х17 | Strength, thermal conductivity, good machinability, corrosion resistance | Utensils for heat treatment of products, including steam |
| 439 | 08Х13 | Possibility of use in various operating conditions | Alloy for mass use - production of refrigerators, sinks, washing machines |
Table of stainless steel grades used in the food industry
It is impossible to imagine modern life without anti-corrosion steel. The development of such an alloy has made it possible to make a qualitative breakthrough not only in metallurgy, but also in many other areas. Stainless steels differ from classical ones in that in addition to iron and carbon, they also contain chromium. It is the addition of chromium that gives the alloy anti-corrosion properties.
Stainless steel products are very diverse. You can find a wide selection of products from any manufacturer. For example, high-quality products, as confirmed by numerous reviews, can be ordered in the BSM - Metal online store.
AISI stainless steel marking
AISI marking is increasingly appearing not only on steel products from overseas, but also on Chinese, Russian, European and other products. This classification system takes its name from its birthplace, the American Iron and Steel Institute. The classifier was liked by consumers, manufacturers, and traders.
Classification
The grade of carbon and alloy steel is presented as a four-digit expression. The first digit in it indicates the main alloying component. The second digit identifies the secondary alloying element. The third from fourth digits indicate the carbon content.
- 1ZZZ–C
- 2ZZZ –Ni
- 3ZZZ –Cr+Ni
- 4ZZZ –Mo
- 5ZZZ–Cr
- 6ZZZ - Cr+V
- 7ZZZ –W
- 8ZZZ –Ni+Cr+Mo
- 9ZZZ –Si+Mn
The letter L at the end of the marking indicates reduced carbon content. The same letter in the middle of the marking indicates alloying of the alloy with lead to improve the mechanical properties of steel processed on machines. N at the end of the marking means nitrogen treatment to increase tensile strength, all other things being equal. The letter B in the middle of the marking is boron doping.
Modern industry identifies at least 150 brands by AISI. Let's look at the key, popular steel grades and where they are used.
300 series (family of chromium-nickel alloys)
- 301 - suitable for products with high ductility, characterized by rapid hardening under mechanical influence. Wear-resistant, increased fatigue strength
- 304 is the most widely used grade, which has found application in almost all industries
- 310 – heat-resistant, with the ability to work in aggressive environments at high temperatures (1000 degrees Celsius in oxidative, up to 10,000 in reducing). 310S is suitable for furnace elements in contact with high temperature gases and condensate
- 316 is a steel that holds second place after 304 in terms of application. A favorite brand for the production of equipment for food processing, for surgical instruments, units, modules operating in salt water. Resistance to pitting corrosion
- 321 - for the needs of chemists, the oil industry, welding equipment requiring use at temperatures up to 800 degrees
400 series (ferritic and martensitic steels)
- 405 Ferrite Matrix Welded Products
- 408-heat resistant
- 409 is the most affordable grade of stainless steel, used for car exhaust systems
- 416 - Easily processed on automatic machines due to the additional sulfur
- 420 - the main purpose of making cutlery, excellent polishing
- 430-ferritic matrix, can be processed by pressure, is resistant to corrosion, used in automotive finishing
- 440 - used for high-quality cutlery, a higher amount of carbon allows knives made from this steel to remain sharp longer, with proper heat treatment
Episode 500
- The 500 series contains chromite heat-resistant steel grades.
Episode 600
600 series - was originally created for patented steel grades that do not fall under classification. Today the subsection looks like this:
- 601-604 – martensitic low-alloy
- 610-613 – martensitic secondary hardening
- 614-619 – martensitic with chromium
- 630-635 – half-austenitic with compacted martensite. Used for pipes, pumps, valves. Corrosion resistance is close to 304
- 650-653 – austenitic steels operating under large temperature differences
- 660-665 – austenitic heat-resistant.
The information presented on this page will help you select the required grade of stainless steel in accordance with its characteristics and capabilities. Steel grades are classified according to two main classifiers GOST5632-2014, AISI. Examples explain the marking of stainless steels and alloys. The areas of application of key and popular brands in manufacturing industries are given.
Physical properties
Stainless steel has gained high popularity not only due to its anti-corrosion properties, but also due to its variety of physical properties. Modern corrosion-resistant steels are produced by adding various impurities to the steel.
The physical properties of the finished steel depend on the amount and type of impurity. It should be noted that some grades of stainless steel are susceptible to corrosion after a long period of use. This is due to the composition, that is, the addition of this or that metal. Such an alloy has other advantages that eliminate susceptibility to oxidation.
It is necessary to highlight the main physical properties of stainless steel, which qualitatively distinguish it from a number of other metals. These properties include:
- High strength. Products made from stainless steel are characterized by increased strength in comparison with analogues. Due to its resistance to physical stress, the products are not damaged and do not lose their original shape. High-quality steel remains reliable for more than ten years.
- Resistance to aggressive external environment. Such steel is practically not subject to changes due to environmental conditions. This allows you to maintain the performance properties of the product for a long time.
- Heat resistance. Stainless steel products are resistant to high temperatures, even when exposed to open fire. Also without changing shape, size and properties under significant temperature changes.
- Environmental friendliness. Anti-corrosion properties prevent the oxidation process. In addition, the material does not contain harmful components, therefore it is widely used in the food industry.
- Anti-corrosion properties. The main property that such steel has is that it prevents rust. Moreover, the alloy does not corrode even after exposure to acids or alkalis.
- Appearance. The appearance of stainless steel products is qualitatively different from items made of other materials. Steel has a clean, shiny appearance that does not change after a long period of use.
- Compliance. Such an alloy is easy to process, and making an object of the desired shape from it is not difficult.
The choice of stainless steel with certain physical properties depends on the purpose of its use. Today, a variety of components for the production of stainless steel allows you to create a material with the necessary characteristics.
Most popular brands:
GOST 20Х13 (AISI 420, DIN 1.4021)
– stainless steel with a martensitic structure, cannot be welded, is not prone to temper brittleness, and does not form internal defects during the production process. Used for the manufacture of measuring and cutting tools, springs, leaf springs.
GOST 12Х17 (AISI 430, DIN 1.4016)
– ferritic stainless steel heat-resistant grade, does not contain nickel. Characterized by good anti-corrosion resistance in moderately aggressive chemical environments and high temperatures.
GOST 12Х18Н9 (AISI 304, DIN 1.4301)
– a heat-resistant, corrosion-resistant alloy used in welded structures in contact with aggressive environments. It is used for sheet parts, welded equipment, heat exchangers, pressure devices.
GOST 08Х18H10 (AISI 304H, DIN 1.4948)
– an austenitic type of heat-resistant, corrosion-resistant alloy, used for the production of rolled pipes, components and assemblies for the chemical and engineering industries, heat exchangers, and industrial tanks.
GOST 03Х18H11 (AISI 304L, DIN 1.4306)
– chromium-nickel grade is used for the production of equipment, tanks and pipelines for the chemical industry, in the production of nitric acid and other aggressive substances.
GOST 08Х18H10Т (AISI 321, DIN 1.4541)
– a stainless, heat-resistant and heat-resistant alloy, non-magnetic, resistant to oxidation and has good weldability without preheating. It is used as food and technical stainless steel for the production of rolled sheets and pipes, welded equipment, the manufacture of containers, tanks, tanks and equipment in the chemical and oil and gas industries.
GOST 03Х17H14М2, 03Х17H14М3, (AISI 316, 316S, 316L)
– non-hardening austenitic grade, areas of application – welded parts, equipment for the pulp and paper and chemical industries, boiler bodies, tanks and installations for the coal industry.
GOST 08Х17H13М2Т (AISI 316Ti, DIN 1.4571)
– structural heat-resistant heat-resistant stainless alloy is used for fasteners and welded structures in various industries.
GOST 20Х23H18 (AISI 310S, DIN 1.4845)
– heat-resistant and heat-resistant austenitic stainless steel used for the manufacture of forgings, clamps, combustion chambers, fasteners and boiler elements, used pipes, couplings.
When choosing stainless steel, you should take into account the operating conditions of the metal, the expected load, and the necessary additional properties of the product. If you are in doubt about how to choose the right stainless steel, it is better to contact a specialist. Leave a request on the website, and our managers will give recommendations on selecting the optimal grades of stainless alloys for the given operating conditions.
Chemical composition
The chemical composition of stainless steel depends on the type and grade of the alloy. The main features that characterize stainless steel are the presence of at least 10.5% chromium and low carbon content. Carbon is very important in steel making as it gives the required strength. The percentage component of which in the anti-corrosion alloy should not exceed 1.2%.
Stainless steel may also contain Titanium, Phosphorus, Molybdenum, Sulfur, Nickel and Niobium. Depending on the chemical composition, stainless steel is divided into several types.
The most widely used is stainless steel of group A2. Group A2 contains 10% nickel, 18% chromium and 0.05% carbon. Most of it is occupied by the base, namely iron with accompanying components.
The composition of steels in this group includes 0.05% carbon, 2% molybdenum, 12% nickel and 17% chromium. Due to the presence of molybdenum in the composition, the alloy is resistant to acid, so the name “acid-resistant” is often applied to it.
Anti-corrosion steels of group A, due to their chemical composition, are easy to weld. That is why this type is widely used in industry. From such steel it is possible to produce parts of almost any shape, with a strong connection of the component parts.
Particular attention in production is paid to steel for the food industry. In this case, corrosion-resistant steel should not contain foreign components that can negatively affect the taste of products, as well as impurities hazardous to human health.
The resistance of steel to corrosion depends on the amount of chromium. The larger its component, the more stable the alloy. Classic stainless steel used under normal conditions contains no more than 13% chromium. To withstand an aggressive environment, the proportion of chromium must exceed 17%. This corrosion-resistant alloy is suitable for use in acidic environments.
Highly resistant alloys retain their properties even in nitric acid of 50% saturation. For resistance against stronger acids, the percentage of nickel in the composition is increased and other components are added in small quantities.
Read also: Angler cutting depth 230
What is included in stainless steel?
chemical composition of stainless steel
A few words about the “ingredients” used in the “cooking” of stainless steel. Or more precisely about alloying elements and their properties. By the way, steel is divided according to the degree of alloying. Austenitic corrosion-resistant steels are classified as high-alloy steels, since the total mass fraction of alloying elements is at least 10%, and the iron content is more than 45%. Let's continue the story about austenitic high-alloy chromium-nickel stainless steel 08Х18Н10, also known as AISI 304, which has alloying elements totaling approximately 28% (18% chromium and 10% nickel). This stainless steel is an alloy in which chromium (Cr) with nickel (Ni) and several other elements are added to iron (Fe) and carbon (C) during smelting. Carbon is responsible for hardness and strength, reducing toughness and ductility. A high carbon content will begin to reduce the cold brittleness threshold and can lead to difficulty welding the metal. Directly in imported stainless steel AISI 304, in contrast to its domestic counterpart, the percentage of carbon is much lower. Chromium in the alloy plays the role of the main “defender” in the fight against corrosion caused by exposure to aggressive environments and various temperatures. Since, thanks to chromium interacting with oxygen, a thin passive film of chromium (III) oxide Cr2O3 is formed due to the adsorption of oxygen occurring on the surface without destroying the crystal lattice of the original metal. This passive film, uniform in composition and evenly distributed over the entire surface of the metal, contributes to the appearance of stainless properties. Chromium, interacting with nickel, provides a stable austenitic structure, which contributes to high ductility, hardenability, good stampability and weldability of products. Nickel increases corrosion properties and prevents metal grain growth when heated. Chromium also increases the heat resistance of nickel, which, in turn, lowers the threshold of cold brittleness, which allows the use of stainless steel 08Х18Н10 in the temperature range from cryogenic -196 °C to high 800 °C. At temperatures above this value, metal oxidation occurs, accompanied by scaling and decarburization of the steel with complete volatilization of the protective passive film.
Speaking about the contact of AISI 304 stainless steel with food, I would like to note the influence of chromium and nickel. The combination of these two components in the alloy increases the corrosion properties and allows the use of products in aggressive environments. Although each product on store shelves has its own acidity levels, the acidic environment formed during the cooking process when interacting with stainless steel, even under the influence of temperatures during heat treatment of products, becomes insufficiently aggressive to affect or damage the integrity of the protective passive film layer , with which the steel is coated. And this, in turn, prevents the release of any harmful impurities from the metal that can interact with products. Therefore, steel can come into contact with food without any consequences.
Classification of stainless steels
The classification of stainless steels varies among countries, but has common principles. Stainless steel marking is carried out depending on the chemical composition, properties and internal structure of the finished material. Based on this, steel is divided into the following types:
- Ferritic. This group of steels is characterized by a high chromium content, usually more than 20%. Therefore, this type is sometimes called chromium. This chemical composition contributes to high resistance to aggressive external environments. Alloys of this group have magnetic properties. Ferritic steels are relatively cheap and are widely used in industry, second only to austenitic steels.
- Austenitic. A group of anti-corrosion alloys that are characterized by a high content of chromium and nickel. Due to this, they are distinguished by increased strength and flexibility in comparison with analogues. Also easy to weld and resistant to corrosion. Most widely used in industry. They belong to non-magnetic metals.
- Martensitic. A special type of stainless alloy. It is characterized by increased strength and wear resistance. They are not exposed to high temperatures, and at the same time contain a minimal part of harmful components that do not emit vapors during intense heating. This group includes heat-resistant, corrosion-resistant steel.
- Combined. A special type of steel that combines the properties of the above groups. Such innovative steels are developed individually depending on the properties required by the customer. Today, austenitic-ferritic and austenitic-martensitic steels are distinguished.
Stainless steel parts
In turn, grades of stainless steel of the austenitic group are divided into 4 types:
- A1 is steel that contains a significant amount of sulfur, which is why it is more susceptible to corrosion than others.
- A2 is the most widely used grade. Easily weldable without loss of physical properties. Frost-resistant, but susceptible to corrosion in an aggressive acidic environment.
- A3 is a derivative of A2, but with the addition of stabilizing components. It is characterized by increased resistance to high temperatures and acidic environments.
- A4 – alloy with the addition of molybdenum (up to 3%). Characterized by resistance to acidic environments. Widely used in shipbuilding.
- A5 – similar to the A4 brand. It differs only in the ratio of stabilizing components. Manufactured for increased resistance to high temperatures.
Types of stainless steel are not limited to the above types. Since even the slightest changes in the percentage of components can significantly affect the properties of steel.
Steel marking - the meaning of digital and letter indices
Knowing the designation of letter indices and the semantic meaning of the numbers used in steel marking, one can draw conclusions about the need for the proposed grade for a specific purpose, without even looking in the reference book. Overpay for the titanium contained in the alloy if you do not need the high fire-resistant properties acquired by alloying with this expensive metal.
Some letter indices can change the designating element, depending on its location in the marking. Consider the correspondence of letter indices:
- A (at the beginning of the marking) – S
- A (in the middle of the marking) – N
- B – Nb
- B – W
- G – Mn
- D – Cu
- E–Se
- K – Co
- M – Mo
- N – Ni
- P–P
- P–B
- C – Si
- T – Ti
- F – V
- X – Cr
- C – Zr
- Yu – Al
- h – REM
The amount of each of them in the alloy is determined by the numerical value following the letter indicating the element. Expressed as a percentage. In cases where an individual element is small, less than 1%, the number is not placed after the letter index. Carbon, as an important element, is located in front of the marking, but is expressed in hundredths of a percent.
FeNi and Ni alloys are marked only with letter indices. The exception is the number after nickel (mass fraction) and carbon (FeNi only).
If the steel was produced using special smelting methods or remelting methods, this is indicated with a hyphen after the marking. Such special methods and methods include various methods of vacuum remelting, electron beam melting, treatment with slags of synthetic origin, and others. The total number of specific methods for obtaining the required grade of alloy is specified in the standard: 24.
Let's look at examples of deciphering the markings of stainless steels 05Х12Н2М and 04Х14Т3Р1Ф-ВД. 05Х12Н2М contains 0.05% carbon, 12% chromium, 2% nickel, and up to 1% molybdenum content. 04Х14Т3Р1Ф-ВД stands for: carbon 0.04, 14% chromium, 3% titanium, 1% boron, vanadium less than 1% percent, obtained by vacuum arc remelting.
Scope of application of stainless steels
Since their development, corrosion-resistant steels have been used only in high-tech production in such areas as aircraft manufacturing, nuclear energy, petrochemical production and mechanical engineering. Today, stainless steels are widely used in various areas of our lives.
Stainless steel car detail
Let us highlight the main areas of use of stainless alloys:
- Mechanical engineering. Stainless steel is widely used for the production of cars, industrial machines and various units. Ferritic and austenitic types are commonly used.
- Chemical industry. The chemical industry is accompanied by the use of aggressive substances, the maintenance of which requires special equipment. Austenitic alloys are used for its production. Production tanks, pipes and vessels are not exposed to chemicals and do not lose their performance properties.
- Energy. In the electrical power industry, only high-strength materials are used, since the strength and reliability of working units are of particular importance.
- Pulp and paper industry. Almost all equipment in this area is made of high-quality stainless steel.
- Food industry. There are increased requirements for the production, storage and transportation of food products. Therefore, in the manufacture of equipment, you can only use glass, several types of plastic and stainless steel. This ensures an increased level of hygiene.
In the food industry, an alloy containing a small number of components is usually used, since the equipment is not exposed to ultra-high temperatures and aggressive substances. Frost-resistant materials are used for refrigeration units.
- Aerospace sector. Special types of stainless steel began to be used to build airplanes, rockets and spaceships.
- Construction. Stainless steel is widely used in construction and design. Such sheets are scratch-resistant and do not leave hand marks.
Corrosion-resistant steels are also used in many fields, due to the variety of types and properties.
If you find an error, please select a piece of text and press Ctrl+Enter.
Corrosion-resistant steels are metal alloys that have increased resistance to corrosion in various climatic and atmospheric conditions, as well as in salt and fresh water, in some gas areas, acids and alkalis. Next, we will describe in detail the properties and characteristics of the main grades of anti-corrosion steel.
Food grade stainless steel according to GOST
There is no official concept of food grade or technical stainless steel. This is the name given to any brand that is suitable for making cookware. Requirements for products intended for contact with products are set out in GOST 27002-86.
The list of possible alloys includes grades with an amount of carbon of at least 12%, chromium of at least 13%, possible presence of nickel in an amount of 5-13%, and molybdenum of about 2%.
Their selection is influenced by the following criteria:
- will the cookware be used for cooking?
- how long the contact is expected to last.
Also, there are no alloys that are used only for the manufacture of dishes, cutlery, etc. Dishes, pipes and tools can be made from the same grade. In this case, the final thermomechanical treatment can be applied in the same way.
Preferably, 12X13 stainless steel is used to make utensils that do not come into contact with food for a long time and are not subject to shock or heat.
Grade 12Х18Н10Т is a classic version of food-grade stainless steel, and since it is used in the mass production of not only tableware, its second name is medical steel.
Steel grades for food supplies
The classification of brands is carried out according to series, which indicate the internal structure after the final thermomechanical processing of the product.
There are 3 series that determine the properties of stainless steel:
Episode 400
— martensitic-ferritic stainless steel. They are distinguished by high manufacturability, i.e., good workability by pressure (rolling, stamping), and weldability. These grades contain 8-40% (on average 12%) carbon, and the main and only alloying element is chromium, contained in an amount of 13% (not less).
Stainless steel with a chromium content of 13-17% has a number of disadvantages: they are classified as weakly rusty, since with prolonged contact with water or mildly aggressive acids, pitting corrosion may appear on the surface.
This entire series cannot be used for products exposed to low temperatures (below -40 ºС) and shock loads.
Affordability and high machinability make these brands in demand for the manufacture of technical parts, structural elements, and pipelines. Cutlery (forks, spoons), coasters, dishes, and candlesticks are no exception.
| Russian classification | European equivalent |
| 08Х13 | AISI 409 |
| 12Х13 | AISI 410 |
| 20X13, 40X13 | AISI 420 |
12X17AISI 430 - used for the manufacture of dishes, cutlery, etc., limitedly used for contact with food and not intended for heat treatment.
Episode 300
— austenitic, austenitic-ferritic and austenitic-martensitic stainless steel. All grades in this series have increased corrosion resistance at temperatures up to 600 ºС (with the addition of alloying elements the temperature limit rises to 800-1100 ºС), and strength.
Nickel is added as the second alloying element in an amount of 5-13%, which contributes to obtaining an austenitic structure, and up to 2% molybdenum and/or 1% titanium are added to increase strength.
The series begins with a universal stainless steel, which is known in all spheres of human activity:
- 08Х18Н10 - chromium-nickel. The most commonly used steel is in the food industry.
Due to its complete inertness to water and mildly aggressive acids, it received the name “food grade”; if the carbon content in this alloy is increased to 12%, the name will sound like “medical steel”.
Intensively used in the chemical and medical industries.
- 10Х17Н13М2 - chromium-nickel-molybdenum alloy.
The additive of 2% molybdenum makes it durable and wear-resistant. It is also used for products in contact with food, but at high temperatures and pressure. These can be steam boilers, pipe systems for transporting liquid media. For industry, gas turbines are made from this alloy.
- 10Х17Н13М2Т - the previous alloy with the addition of titanium.
Titanium increases the operating temperature to 800-1100 ºС and the ability to work in aggressive environments with chlorine. Used in critical systems for the production of seamless pipes, as well as shut-off and connecting fittings for them.
| Russian classification | European equivalent |
| 10Х17Н13М2 | AISI 316 |
| 10Х17Н13М2T | AISI 316 T |
| 12-08Х1810Т | AISI 321 |
Episode 200
- with a predominance of only the austenite structure. Its properties are similar to both previous series, but the cost is much cheaper than the 300 series.
12X15G9ND - in this brand (it is the only one so far) nickel and molybdenum are replaced by two elements balanced in relation to each other: manganese and copper. High technology and low cost (relative to chromium-nickel brands) make this series stand out.
| Russian classification | European equivalent |
| 12X15G9ND | AISI 201 |
3 Chromium-nickel alloys - the basis of construction and production
This type of anti-corrosion steel is the most common in modern industry and production. Today, more than 50 grades of chromium-nickel alloys are known, from which hot-rolled pipes, long and sheet metal, profiles, fittings, angles, and channels are made. In addition, such steel is widely used in the chemical, energy, aircraft and automotive industries. Grades of chromium-nickel steels can be divided into several classes:
- austenitic with low carbon content and the addition of stabilizing elements;
- acid-resistant with various additives;
- heat-resistant with a high content of nickel and chromium (more than 20%);
- austenitic-martensitic and austenitic-ferritic with an average content of nickel and chromium;
The main alloys of this type are grades ОХ18Н9, ОХ18Н10, 2Х1Н9, ОХ18Н11 and other types 18-8 (that is, 18% Ni in the alloy composition), stabilized with titanium and other alloying elements. Chromium-nickel austenitic steels are widely used for furnaces, heat turbines, and outlet manifolds. They can be worked in conditions of high external aggressiveness of the environment and subjected to short-term heating to a temperature of 600-650 degrees without additional heat treatment of the surfaces.
Chromium-nickel anti-corrosion and heat-resistant steels with the addition of silicon or boron (Ox23N18, X23N18, X25N16) are used for the production of heat-resistant sheets, tapes, tubes, wires, which are used for various equipment that operates at temperatures above 850 degrees. In the structure of chromium-nickel and nickel steels there is a certain amount of ferrite, which decreases with increasing content of elements such as manganese, silicon, molybdenum, and increases when nitrogen, boron, copper or nickel are introduced into the composition in a large percentage. All these properties reflect steel grades of a similar type and are determined using special content tables.
Corrosion-resistant (stainless) steels and alloys
BASICS OF THE THEORY OF CORROSION
The destruction of metals and alloys as a result of chemical or electrochemical action on their surface of an external aggressive environment is called corrosion .
Corrosion, as a rule, is accompanied by the formation of corrosion destruction products on the metal surface. For example, on the surface of iron alloys, as a result of corrosion, rust is formed, which has a brown color. In some individual cases, metal corrosion is not accompanied by the formation of such noticeable destruction products, and then its appearance is quite difficult to detect.
Corrosive destruction is the result of the interaction of a metal with the external environment and the intensity of its development depends on the properties of the metal itself, as well as on the nature of the environment. Most metals, being resistant in some environments, are quite easily destroyed when interacting with other environments. For example, copper alloys are stable in humid atmospheres, but are highly susceptible to corrosion if even small amounts of ammonia are present in the atmosphere; Tantalum and titanium at room temperatures are very stable in many aggressive environments, but they acquire high chemical activity when heated above 600 ° C.
There are several types of corrosion: continuous or uniform, when the entire surface of the product is exposed to corrosion; point or local, if corrosion develops in individual small areas; intergranular corrosion (ICC), when corrosion spreads deep into the product along the grain boundaries; Stress corrosion is the occurrence of corrosion cracks due to the simultaneous impact of tensile stresses and an aggressive environment on the metal.
Corrosion can occur as a result of purely chemical reactions with the environment, as well as due to electrochemical processes occurring at the interface between the metal and the external environment. The largest amount of metal is destroyed as a result of electrochemical corrosion.
Electrochemical corrosion is the destruction of metals and alloys when exposed to electrolytes. This type of corrosion is characterized by the flow of electric current, the transition of atoms to an ionized state and other electrochemical processes.
The most common electrolytes in practice are aqueous solutions of salts, acids and alkalis. Thus, electrochemical corrosion includes corrosion of metal containers, pipelines, machine parts and parts of stationary structures under the influence of acids, sea, river, ground and other waters. The most common is atmospheric corrosion.
If there are two metals with different electrode potentials in the electrolyte, then the metal with a more negative electrode potential (anode) continuously releases ions into the solution (dissolves), and the resulting excess electrons continuously flow into the metal with a less negative electrode potential (cathode). The cathode in the contact pair is not destroyed; electrons from it are continuously removed into the external environment.
All metals can be arranged in a row in descending order of their electrochemical potential:
Metal…………………. Au Ag С u Η Ni Fe Ζ n Α l
Electrode potential, V +1,42 +0,80 +0,34 0 -0,23 -0,44 -0,76 -1,66
In technical metals and alloys, which are polycrystalline bodies, the microstructure consists of grains of one or more phases, non-metallic inclusions, etc. These different structural components, which have different physical and chemical properties, upon contact with the electrolyte acquire electrode potentials of unequal magnitude and sign and some of them will become anodes, and others - cathodes. Thus, industrial metals and alloys, when exposed to electrolytes, can be considered as multielectrode elements consisting of a huge number of microscopically small corrosive galvanic pairs - microgalvanic pairs. The more the electrode potentials of the phases in the alloy differ, the faster its corrosion destruction occurs (in particular, dendritic segregation is precisely why the resistance to electrochemical corrosion decreases). It follows that either very pure metals or alloys that have a uniform (homogeneous) solid solution structure can have high corrosion resistance.
The passive state is the state of a metal (alloy) when it exhibits increased corrosion resistance (even practically stops corroding) in an aggressive environment. The opposite state, when the same metal corrodes, is called the active state.
Experimental data show that the transition of a metal from an active to a passive state is associated with an increase in its potential. For example, iron in its normal state has an electrode potential of -0.4 V; in a passive state, its potential can rise to +1.0 V.
Effect of doping. There are two groups of corrosion-resistant metals. Some metals resist corrosion well due to their low chemical reactivity. Others, being by their nature active elements, acquire high chemical stability due to the phenomenon of passivity. The first group includes platinum, palladium, gold, the second group includes chromium, titanium, aluminum, etc. To increase the corrosion resistance of a reactive metal, alloying elements are introduced into it.
When a metal is alloyed with another, more noble metal, the potential of the alloy initially remains virtually unchanged. But when a certain concentration is reached, a jump in potential occurs and the corrosion resistance of the alloy in a given environment also increases abruptly, and boundaries (thresholds) of stability appear.
It was experimentally established that such sharp changes in stability occur when the ratio of atoms of the alloying element to the alloyed element is a multiple of 8, i.e. n/8, where n is an integer 1, 2, 3... This corresponds to 12.5; 25; 37.5...% (at.) alloying element.
The appearance of stability boundaries is explained by the fact that when the alloy interacts with an aggressive environment, some of the atoms of the base metal go into solution, and the remaining atoms of a more noble or easily passivating metal form a kind of barrier on the metal surface. This barrier consists either of the noble metal atoms themselves, or of protective shielding films.
In more active environments, a higher concentration of a stable element is required, i.e. in this case, the limits of stability arise at a higher value of the number n.
The stability limit is also observed in alloys in which one of the components has the ability to self-passivate. This boundary is also observed in systems when one of the components in a given aggressive environment forms protective shielding films from insoluble compounds.
The corrosion resistance of steel can be increased if, firstly, the carbon content is reduced to the minimum possible amount and, secondly, an alloying element that forms solid solutions with iron is introduced in such an amount that the electrode potential of the alloy increases abruptly.
Steel that is resistant to atmospheric corrosion is called stainless steel. A steel or alloy that has high resistance to the corrosive effects of acids, salts, alkalis and other aggressive environments is called acid-resistant.
CHROME STAINLESS STEEL
Chromium is the main alloying element that makes steel corrosion resistant in oxidizing environments. The corrosion resistance of chromium stainless steels is explained by the formation of a protective dense passive film of Cr2O3 oxide on the surface. Such a film is formed only when the chromium content is more than 12.5% (at.). It is at this chromium content (n=1) that the potential changes abruptly from -0.6 to +0.2 V.
Iron and chromium form a continuous series of solid solutions (See iron - chromium diagram). Thanks to this, it is possible to obtain steel with a high chromium content in solid solution. Chromium is not a scarce metal, its cost is relatively low, so chromium steels are the cheapest stainless steels. These steels have a fairly good set of technological properties. Carbon in stainless steels, including chromium steels, is an undesirable element, since, by binding chromium into carbides, it thereby depletes the solid solution of chromium, reducing the corrosion properties of the steel. In addition, carbon expands the region of the γ-solid solution, facilitating the formation of a two-phase state (Fig. 1).
Rice. 1. The influence of carbon on the position of the γ region - solid solution on the iron - chromium diagram
With a higher chromium content, the σ phase will be present in the steel.
The higher the chromium content, the higher the corrosion resistance of chromium steels. Currently, three types of chromium steel are smelted: 1) containing 13% Cr; 2) 17% Cr- 3) 25-28% Cr.
Steels 08X13 and 12X13 have increased ductility and are used for the manufacture of parts subject to shock loads (turbine blades, cracking unit fittings, household items, etc.).
From steels 30X13 and 40X13, which acquire a martensite structure after heat treatment, measuring and medical instruments, springs and other corrosion-resistant parts that require high hardness or strength are made.
Steels containing 17 and 25-28% Cr are classified as ferritic steels. They have higher corrosion resistance compared to X13 steels. When heated above 850° C, ferritic steels tend to grow grains and their ductility decreases. To obtain a single-phase structure and reduce the tendency to grain growth and MCC, titanium and niobium (08X17T, 15X25T) are added to these steels. Strength increases, ductility remains sufficient, and the properties of welds improve. These steels are used for the manufacture of equipment operating in such aggressive environments as fuming nitric acid, phosphoric acid, and make equipment in the chemical and food industries corrosion-resistant. Heat exchangers for hot nitrous gases, pipelines and tanks for acids, etc. are made from 12X17 steel.
The introduction of molybdenum (12Х17М2Т) makes the steel resistant even to organic acids (acetic, formic). Ferritic grade steels are not susceptible to stress corrosion.
For the manufacture of ball bearings operating in aggressive environments, steel 95X18 (0.9-1.0% C, 17-19% Cr) is used.
All chromium steels are subjected to hardening at 1000-1100°C followed by tempering (for ferritic steels - at 700-750°C, martensitic class 200-250°C).
Ferritic steels do not undergo transformations when heated, so heat treatment is carried out to obtain a more homogeneous solid solution structure, which increases corrosion resistance.
CHROME-NICKEL STAINLESS STEEL
Nickel is one of the metals that easily acquire passivity, although its passivation ability is less than chromium and molybdenum. Adding nickel to iron in an amount of 1/8 mole dramatically improves the corrosion resistance of the alloy in sulfuric acid. At a nickel concentration of 2/8 mole, the corrosion resistance increases even more.
Iron-nickel phase diagram. Nickel is an austenite-forming element that greatly reduces the critical points of the γ-να transformation. Nickel also has this effect when it is introduced into chromium steels. Therefore, steel containing 18% Cr and 9% Ni has an austenite structure at room temperatures (see Fig. 2).
Rice. 2. Structural diagram of stainless steels
Stainless steels with an austenitic structure have higher corrosion resistance,
better technological properties compared to chromium stainless steels, in particular, better weldability. They retain strength to higher temperatures, are less prone to grain growth when heated, and at the same time, austenitic steels do not lose ductility at low temperatures. Like chromium steels, chromium-nickel steels are corrosion-resistant in oxidizing environments. The main element that increases the potential of iron is also chromium, so its content should be >13%. Nickel only further increases the corrosion resistance of steels.
The composition and properties of chromium-nickel stainless steels are given in GOST 5632-72. In Fig. Figure 2 shows a structural diagram that allows you to determine the structure of steel depending on its composition.
Chromium-nickel steels, depending on composition and structure, are divided into steels of austenitic, austenitic-martensitic and austenitic-ferritic classes.
The lower the carbon content, the higher the corrosion properties of stainless steels. The carbon contained in chromium-nickel steels can be in solid solution, as well as in carbides or carbonitrides of varying degrees of dispersion. Cr23C6 carbides are predominantly formed, and they are formed already at a carbon content of slightly more than 0.04% (0.04% C is the solubility limit of carbon in austenite alloyed with nickel). If steels contain nitrogen (for example, X17AG14 steel), then carbonitrides of the Me23(C,N)6 and Me(C,N) type can be formed.
Most chromium-nickel stainless steels belong to the austenitic class: 04Х18Н10, 12Х18Н9Т, 09Х14Н16Б, 08Х10Н20Т2, etc. These steels are ductile, easy to weld, have increased heat resistance, and are corrosion-resistant in many environments with moderate activity. Steel 12Х18Н10Т is the cheapest and therefore most often used.
For greater homogeneity, chromium-nickel steels are quenched at 1050-1100 ° C in water. In this case, σΒ = 50-60 kgf/mm2 and δ = 35-45% are obtained. These steels are strengthened by cold plastic deformation.
Additional alloying of chromium-nickel steels with molybdenum and copper increases their corrosion resistance and acid resistance (03Х16Н15МЗ, 03Х17Н14М2). Sometimes titanium and aluminum are introduced into these steels in small quantities, which, forming dispersed intermetallic compounds of the Ni3(Ti,Al) type, strengthen the austenite (08Х17Н13М2Т, 08Х17Н15МЗТ).
Steel 06ХН28МДТ (0.06% C; 22-25% Cr; 26-29% Ni; 2.5-3% Mo; 2.5-3.5% Cu and 0.5-0.3% Ti) has high corrosion resistance, it is used in highly aggressive environments (diluted sulfuric acid, etc.). This steel, after quenching at 1100″C in water, has an austenite structure with a small amount of carbonitrides. After short-term heating to 500-900° C, it does not show a tendency to MCC.
Nickel is a fairly expensive and scarce metal, so stainless steels are created with a lower nickel content. To do this, other austenite-forming elements are introduced into the composition of stainless steels, for example, manganese and even nitrogen (steels 10Х14Г14Н4Т, 15Х17AG14, 10Х14AG15, etc.).
Austenitic-martensitic steels (transitional steels) have lower corrosion resistance compared to austenitic steels, but exceed them in strength (σΒ = 120-130 kgf/mm2). Transitional class steels include steels 09X15N8Yu, 09X17N7Yu, 08X17N5MZ, 20X13N4G9, etc.
The heat treatment regime of these steels is characterized by great complexity: hardening, cold treatment, tempering - aging. In Fig. Figure 3 shows the effect of various types of heat treatment on the strength of stainless steels of various classes. Transitional class steels receive the greatest strengthening. Such steels are used to create lightweight structures with high resistance to corrosion damage.
Rice. 3. The influence of heat treatment on the strength of stainless steels:
1 - hardening; 2 - hardening and cold treatment; 3- hardening, cold treatment, tempering (aging)
Austenitic-ferritic steels are proposed as substitutes for chromium-nickel steels of the X18N8 type in order to save nickel. This class includes steels 12Х21Н5Т and 08Х22Н6Т. Austenitic-ferritic steels at room temperatures have higher strength and hardness than steel type 18-8, but their ductility and toughness are lower. These steels do not have stable properties: their properties depend on the ratio of ferrite and austenite phases, which in turn depends on the total influence of ferrite-forming (Cr, Ti, Mo, Si) and austenite-forming (Ni, N2, C) elements. With an increase in the amount of ferrite, the heat resistance of steels decreases, the strength increases, and the ductility decreases, but not below 30%. Good technological properties are obtained with the ratio Φ:A=1:1.
Steel 15Kh28AN, which has good mechanical properties, also belongs to this class of steels.
(σΒ = 65–70 kgf/mm2, δ = 11–23%), including in the weld.
Typical heat treatment of austenitic-ferritic steels: hardening from 1000-1150° C and tempering - aging at 500-750° C.
Austenitic-ferritic steels are not subject to stress corrosion cracking: cracks can only occur in the austenitic areas, but the ferritic areas retard their development.
Stainless steels exhibit a special type of corrosion called intergranular corrosion (sometimes also called intergranular corrosion). Such corrosion occurs mainly along grain boundaries and is very dangerous because it does not have any external signs - the metal even retains a metallic luster. At the same time, the strength drops catastrophically, the metallic sound disappears, the metal is so easily destroyed that it can be turned into powder. Intercrystalline corrosion (ICC) develops if a stainless steel product, after hardening, is heated to 500–700° C or if slow cooling is carried out in this temperature range. At the same time, a network of chromium carbides is clearly visible in electron micrographs.
The causes of ICC have been studied for many years and there are several theories explaining the causes of this dangerous phenomenon.
The most accepted is the so-called “impoverishment theory.” It is known that the grain boundary is a transition zone between them.
If the penetration of a dissolved impurity into the intergranular zone reduces the excess energy of the boundaries, the concentration of this impurity in the zone increases. It has been established that carbon reduces the excess energy of boundaries, therefore intercrystalline internal adsorption of carbon occurs along the grain boundaries of stainless steel. Thus, already during quenching, carbon atoms are nonuniformly distributed in the solid solution, their concentration along the boundaries is greater than in the grain. Although chromium carbides are not formed in this case, such an increased concentration of carbon is, as it were, a preparation for their rapid formation. When heated to 500-700° C, chromium carbides Cr23C6 are formed along the grain boundaries. At these temperatures, the diffusion of carbon in solid solution to grain boundaries proceeds faster than that of chromium. Therefore, the formation of carbides consumes not only the carbon reserve present there, but also the carbon diffusing from inside the grains. At the same time, chromium, necessary for the formation of carbides, comes, at the first stages of the process, from the boundaries or from the boundary zones of austenite. As a result, the chromium content in the border zones of grains becomes less than 13% (even up to 6.5%) and they lose their corrosion resistance.
Due to the great danger of the MCC phenomenon, all smelted stainless steels must be checked for susceptibility to this type of corrosion. In this case, samples made of hardened steel are subjected to provocative tempering for an hour at 650° C. After this, the samples are boiled in an aggressive environment and the presence of MCC is determined.
The tendency to MCC in stainless steels can be eliminated: 1) by reducing the carbon content (in steels containing 0.02% C, MCC is not observed); 2) the introduction of elements - titanium or niobium stabilizers, which have a greater affinity for carbon than chromium; 3) using stabilizing annealing (heating the product to 850°C).
Rice. 4. Microstructure of chromium-nickel stainless steel 08Х18Н9 without MCC (a) and with MCC. (b)
When welding in the heat-affected zone, the metal can heat up to dangerous temperatures (500-700 ° C). Therefore, if the steel is prone to MCC, then welded products should not be made from it, or after welding it is necessary to carry out heat treatment, at least annealing to 650°C. In Fig. Figure 4, α shows the microstructure of stainless steel 08Х18Н9 after heat treatment (quenching at 1100° C in water), heating at 650° for an hour and boiling in sulfuric acid for 48 hours (Fig. 4, b).
CORROSION-RESISTANT ALLOYS AND CAST IRONS
In addition to stainless steels, other corrosion-resistant alloys are also used in industry.
For particularly aggressive environments, nickel-based alloys such as Hastelloy (NIMO alloys) are used. The nickel content in these alloys reaches 80%. The second element present in these alloys in large quantities is molybdenum (15-30%). The composition of some alloys is given in table. 1.
Table 1.
Chemical composition (%) of acid-resistant nickel-based alloys such as Hastelloy
| Alloy | WITH | Μn | Si | Cr | Mo | Fe | Other elements |
| Hastelloy A (EI460) | <0,12 | <3 | <1 | _ | 20 — 22 | 18 — 20 | |
| Hastelloy V (EI461) | <0,12 | <3 | <1 | <1 | 26 — 30 | 4 — 7 | 0.3V |
| Hastelloy S (EP375) | <0,12 | <1 | «1 | 15,5— 17,5 | 16 — 17 | 4,5 -7 | 3.75-5.25W |
| Hastelloy D | <0,12 | 0,8 -1,25 | 8,5-10 | <1 | _ | <1 | 3.6-6.5Cu |
These alloys have very high corrosion resistance in environments where, apart from them, only a few metals are stable (for example, in boiling phosphoric acid up to a concentration of 50%, in boiling hydrochloric acid up to 20%, etc.).
Hastelloy alloys have high mechanical properties, which can be improved by heat treatment - hardening + aging at 800° C. In this case, σв = 120 kgf/mm2 and hardness ΗВ = 360.
The disadvantage of alloys is their tendency to MCC, so the carbon content in them should be minimal.
Corrosion-resistant cast irons are resistant to many aggressive environments (and not only oxidizing ones). They are usually heat resistant. Alloy cast irons are cheaper than stainless steels and have good casting properties, so products made from them are produced by casting methods. The chemical composition and properties of acid-resistant cast iron are given in GOST 2176 and GOST 2233 (Table 2).
Chromium cast irons contain 26-36% Cr. The structure of chromium cast iron is a solid solution of chromium ferrite and eutectic carbides. Carbides can also be in a free state, and Cr7C3 carbides are predominantly formed. Chromium cast irons (X34) have high hardness (HB 325-400), resist wear well, but are difficult to machine. Alloys 25X18L and 30X20L are related to steel in terms of carbon content, and cast iron in terms of properties. Their casting and mechanical properties are better than those of X28 and X34, they are less prone to the formation of hot cracks
Table 2.
Chemical composition (%) of corrosion-resistant cast irons and cast steels
| Brand | WITH | Si | Μn | Cr |
| Chrome cast irons | ||||
| X28 X34 | 0,5-1,0 1,5-2,2 | 0,5—0,8 1,3-1,7 | 0,5—0,8 0,5—0,8 | 26—30 32—36 |
| Chrome steels | ||||
| 25X18L 30X20L | 0,20—0,30 0,25—0,35 | <0,8 <0,8 | <0,8 <0,8 | 17—20 20—23 |
| High silicon cast irons | ||||
| S15 S17 F15* | 0,5-0,8 0,3—0,5 0,5—0,0 | 14,5-16 16—18 15—16 | 0,3—0,8 0,3—0,8 0,3—0,8 | _ |
| Nickel cast iron** | ||||
| SCHShch-1 SCh1D-2 | 3,2—3,5 3,2—3,6 | 1,2-1,5 1,5—2,0 | 0,5—0,8 0,4—0,8 | 0,6—0,8 0,4—0,8 |
* Contains 3.5-4.5 Mo.
** Alloy SChShch-1 contains 0.8-1% Ni, alloy SChShch-2 0.4-0.5% Ni.
Chromium cast irons are resistant to oxidizing environments: in nitric acid of any concentration at 20°C and 40% boiling; in concentrated sulfuric acid and other media. Scale resistance is maintained up to 1000-1100° C.
Chromium cast iron is used to make parts and equipment for the nitrogen industry, artificial fertilizers, dies, etc. They are also used as heat-resistant materials - for the manufacture of furnace equipment, grates, combs and blades in furnaces intended for firing.
Silicon cast irons are acid-resistant alloys. Silicon, like chromium, expands the range of existence of ferrite and alloys containing up to 14.5% Si have the structure of a homogeneous solid solution. The carbon content in silicon cast iron is only 03-0.8%; with a higher content, carbon can be released in the form of graphite. Cast irons are smelted with a silicon content of up to 18%, since at a higher content these alloys become brittle and cannot be used. With a sharp change in temperature, cracking is possible. In oxidizing environments, a strong SiO2 film is formed on the surface of products, which is restored in case of mechanical damage.
Products from silicon cast iron are made only by casting, without subsequent mechanical processing (only grinding is possible).
The Φ15 alloy, also called "antichlor", contains 3.5-4.5% Mo. As a result of the addition of molybdenum, the alloy is stable in 10-30% solutions of hydrochloric acid (up to 90 ° C).
Silicon cast iron is used to make centrifugal pumps, acid sprayers, taps, boilers, vats, etc. All silicon cast iron has high oxidation resistance.
Nickel cast irons contain ~1% Ni (see Table 2). These cast irons are resistant to molten salts and concentrated alkali solutions. With increasing nickel content, the corrosion resistance of cast iron increases. The composition of nickel cast iron can be more complex: nickel-silicon austenitic cast iron contains,%: 1.7-2 C; 1.8-3 Cr; 5–7 Si and 16–20 Ni; nickel copper 2-2.8 C; 3-4 Cr; 5-8 Cu; 1.5-1 Si and 12-5 Ni.