What is alloy steel
What kind of miracle is these alloy steels? What is their difference? These are carbon steels, to improve the characteristics of which alloying components are used. The degree of alloying can be different, but even with small additions, the quality characteristics of the material increase significantly.
Production
Alloying of steel is carried out in two ways according to special rules.
The main method is metallurgical. In this process, a certain number of additives are added to the molten metals. After this, additional parameters are set, after which chemical reactions are carried out in accelerated mode.
An additional option for alloying is that additives are applied as a surface layer to begin the mutual penetration of elements.
Chemical composition
Alloying elements contain permanent additives. They are found in every alloy. In addition, there are optional ingredients for alloying. The main components include:
- Iron. The main feature of this malleable metal is its presence in the depths of the earth. In terms of production, it is in second place after aluminum. It easily enters into chemical reactions, so it is easy to fuse. The percentage of iron content can range from 45 to 97-99%, depending on the grade of steel.
- Carbon. It is considered one of the most important ingredients of the composition. Typically the element is added in the amount of 0.1% to 1.4% of the total mass. The more carbon, the stronger the metal.
- Manganese. A useful element related to the basic ones. If its amount is less than 1%, it will not provide serious improvements. It looks beautiful in appearance - it is silver in color, so metal ingots from it have a specific shade. The main benefit is that it helps remove oxygen from the material. In individual compounds, in accordance with GOST standards, it is present in an amount of 11–14% of the total mass. In this case, the steel is demagnetized, becomes more impact-resistant and does not wear out for a long time.
- Silicon is an essential component of the composition, if the alloy contains more than 0.8%, it has alloying qualities. Like manganese, it eliminates unnecessary oxygen. Increases wear resistance, elasticity, and strength of the material under high heat.
Interesting: Types and equipment of metal slitting
Additives also include harmful components. Experts are trying to remove them, but it is impossible to completely remove them. These include:
- Sulfur. Increases the likelihood of cracks appearing on heated metal.
- Phosphorus. Increases the fragility of the material.
- Oxygen, nitrogen and hydrogen have the properties of “loosening” the alloy.
- Oxides and nitrides increase the likelihood of tears.
Another group of elements is random. They appear in containers with mixtures, that is, mixtures of the original components, and do not have useful qualities. They may be harmless, but since the proportion of their content is small, they practically do not affect the structure. These include:
- Copper;
- Zinc;
- Lead;
- Chromium;
- Nickel.
The last group includes special elements. They are used to improve certain properties of the metal. They impart strength to the alloy. These elements are additionally introduced to enhance certain characteristics. These include:
- Titanium;
- Vanadium;
- Molybdenum;
- Tungsten;
- Some others.
Properties and characteristics
The properties and characteristics of alloy steels are very diverse. They depend on additives used as alloying agents in the manufacture of certain types of steel.
Depending on the amount of impurities, the material receives the following properties:
- Strength. This characteristic increases when chromium, titanium, and some other components are added to the alloy.
- Non-corrosive. Increases after adding chromium and molybdenum.
- Hardness. It increases after the appearance of manganese, chromium, and other components in the metal composition.
Important: In order for the alloy to become stronger and more resistant to environmental influences, the composition must contain chromium in an amount of at least 12%.
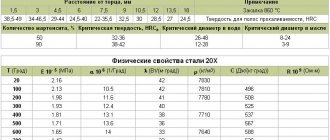
Steel should not change characteristics when heated to 600 C°.
Examples
To learn how to decipher symbols, you need to consider several labeling options:
- U8GA - contains 0.8% carbon.
- St3sp5 is a structural metal that is not alloyed. Often used for the manufacture of metal structures.
- 30ХГСА - contains up to 0.3% carbon. Additional components - silicon, manganese, chromium. The letter A indicates high quality material.
- R6M5F2K8 - high-speed steel. The composition contains about 8% cobalt, 5% molybdenum, 2% vanadium.
- HVG - consists of manganese, chromium, tungsten, the amount of which does not exceed 1%.
Classification of alloy steels
The following classification of types of alloy steels is distinguished.
By quality
Depending on the quality, alloy steels are divided into types:
- Structural;
- Instrumental;
- Having special physical properties.
Useful: Structural and tool alloy steel is used in areas requiring high strength. Types of metal with special properties are corrosion-resistant, resistant to extreme temperatures and harsh chemical environments. These materials include stainless steel.
By number of additives
There are three main types of alloy steel, which are stainless in nature.
- Low alloy. This steel contains 2.5% alloying additives.
- Medium alloyed. It contains from 2.5 to 10% alloying elements.
- Highly alloyed. This includes steel in which the number of alloying components is over 10%. In some varieties the number of such elements can reach 50%.
Interesting: Properties and characteristics of steel 40x
By purpose
According to practical purposes, alloy steels are of two types:
- Mechanical engineering materials are used in the manufacture of parts for various mechanisms and in body structures. They undergo special temperature treatment.
- Construction alloys - usually used in the production of welded metal structures, they are rarely subjected to high temperature processing.
Properties and types of steels
Steel has the following properties:
- Physical: heat capacity, electrical and thermal conductivity, expansion when heated.
- Mechanical: strength, hardness, elasticity, plasticity, viscosity, endurance.
- Chemical: heat resistance, scale resistance, fire resistance, corrosion resistance.
To significantly change the properties of the alloy, alloying elements - other metals and non-metals - are introduced into the steel. This technology was created back in the 19th century. Steels are called alloyed if the proportion of each element is at least 0.1%.
Marking of alloy steels
The exact composition of each alloyed material can be found in reference books. However, you can find out such information if you understand the markings in alloy steel. Alloying elements can be identified by their letter designation. In addition, it is possible to decipher the percentages of various elements in the metal.
Important: The marking is indicated by a letter that shows the purpose of the alloy. The letters indicate the following brands:
Zh, X, E - stainless, chromium, magnetic metals.
I am a stainless steel chromium nickel alloy.
Ш - steel of the ball-bearing variety.
R - cutting metal.
A, Sh - steel with impurities, of high quality.
Explanation of markings
In addition, steel may contain different components. Attached is a table showing how the various metals in alloys are designated:
Important: if a number is indicated before the letter, this is the amount of silicon used; when numbers are shown after the letter abbreviation, the percentage of the indicated substances is meant.
Decoding
Steel is marked according to certain rules. The first numbers indicate the carbon content. For example, 5 indicates 0.05%. If there is no number at the beginning, the amount of carbon is up to 1%.
The letters that follow the first numbers indicate the name of the additional components.
There are different types of steels that differ in technical characteristics and properties. They depend on the presence of additional impurities contained in the composition. To avoid confusion among numerous products made from different types of materials, special markings are used.
Application of alloy steel
Alloy steel is widely used in the engineering industry, production of tools, and building materials.
Automotive parts are made from pearlitic alloys. These include low and medium alloy steel. After annealing, the metal structure allows the material to be processed with cutting tools.
Lightly alloyed steel is used in shipbuilding because the material allows the thickness of the alloy used to be reduced.
Metal with chromium impurity is used for smelting products that are not exposed to lactic and acetic acids, in the manufacture of parts operating in a high-pressure environment. These could be gearbox gears, piston pins and other parts.
Interesting: Highly alloyed steel is used to create parts that are resistant to corrosion. Such components withstand extreme temperatures and can serve at + 1100 C°.
Chemical composition
The quality of such material depends entirely on the amount of carbon in it, since this is one of the main components of its composition. It is also necessary to include iron in its composition. Nickel, chromium, copper, vanadium and other components are added to improve other properties of the raw material.
Now let’s look at how alloying elements affect the properties of the resulting raw material:
- Chromium, like nickel, is responsible for imparting rust resistance. With its help, the well-known stainless steel is obtained; the metal is made harder and stronger.
- Nickel adds not only strength, but also ductility.
- Copper, in addition to corrosion resistance, helps resist various acids.
- Vanadium compacts the structure and makes it fine-grained.
- Manganese is responsible for wear resistance.
- Tungsten maintains the hardness of the material when exposed to high temperatures.
- Silicon gives the metal its elasticity and also makes it magnetic.
- The presence of aluminum adds heat resistance to the resulting material.
How does the structure change when various impurities are added? As a result of their introduction, the crystal lattice is destroyed due to differences in the shape of electrons and atomic quantities. Therefore, the characteristics of alloy steel may fluctuate due to changes in the percentage of elements in its composition. The alloy gains hardness, strength and ductility after heat treatment.
Appearance of alloy steel
This metal usually differs in chemical composition. Therefore the classification will be as follows:
- Low alloyed – the percentage of alloyed additives is no more than 2.5.
- Medium alloyed - impurities are no more than 2.5-10%.
- Highly alloyed - impurities can be more than 10% and increase to 50.
According to the classification, it is divided into: corrosion-resistant steel and heat-resistant steel (withstands above 1000 degrees).
According to chemical decomposition, the following are distinguished:
- scale-resistant (at 550 degrees);
- heat resistant.
There are two main types: alloy and carbon. Let's see what differences they have.
Carbon steel is an alloy containing, together with iron and carbon, silicon and manganese. Sulfur and phosphorus, also present in its composition, are considered negative additives, because because of them, its mechanical properties deteriorate.
Steel comes in low, medium and high carbon grades. The greater the proportion of carbon in such alloys, the less their ductility, but the harder the final material is.
Carbon steel is an alloy of iron with carbon up to 2%. Silicon, sulfur and phosphorus are also added to it. However, the main component is still carbon. The percentage of these elements is approximately the following: iron up to 99.0%, manganese - 03-0.8, sulfur up to 0.06 and silicon up to 0.15-0.35.
The main disadvantages of carbon steel:
- if it has good strength and hardness, then it lacks ductility;
- hardness and cutting ability are lost when heated to 200 degrees, and at higher temperatures strength is also lost;
- low resistance to rust when immersed in electrolyte, in aggressive environments, etc.;
- increased coefficient of thermal expansion;
- weighting of finished products;
- increase in the cost of the final product;
- difficulties in design due to the low strength of such steel.
Alloyed – steel, which, along with conventional additives, contains alloyed elements that significantly improve its quality. These are tungsten, molybdenum, nickel, etc. And also manganese and silicon in significant quantities. Impurities are added during melting. This metal is distinguished by its valuable qualities, which are absent in carbon steel, and is free from its disadvantages.
Welding alloys
Alloy alloys are flexible and are used to make complex mechanisms by welding. Due to the different content of impurities, each type of alloy alloys has its own specific characteristics.
Interesting: At what temperature does lead melt?
Low alloy
These metals are often hardened and weld well, but their seams do not withstand excessive stress. It is necessary to preheat the alloy and cool it slowly to prevent cold cracks from forming.
Medium alloyed
Alloy steels of this type are obtained reliable when components made of tungsten, molybdenum, and vanadium are used. It is necessary to select electrodes with the same substances, but not at the same concentration. The alloy requires protection from overheating, oxidation processes, and hydrogen disease.
Classification by purpose
The classification of steel types by purpose has already been given above. Marking of structural steels includes the following designations:
- Construction - denoted by the letter C and numbers characterizing the yield strength.
- Bearing - designated by the letter Ш. Next comes the designation and content of alloying additives, mainly chromium.
- Instrumental unalloyed - denoted by the letter U and carbon content in tenths of a percent.
- High-speed - denoted by the letter P and symbols of alloying components.
- Unalloyed structural steel has the symbols Cn and a number indicating the carbon content in tenths or hundredths of a percent.
Classification of steel by purpose
The remaining varieties, including tool grades made of alloy steels, do not have special designations other than their chemical composition, so the decoding and purpose of individual types can only be determined from reference literature.
We will advise you on any questions!
Any specialist who deals with metal is familiar with the concept of “steel grade”. Deciphering the markings of steel alloys makes it possible to get an idea of their chemical composition and physical characteristics. Understanding this marking, despite its apparent complexity, is quite simple - it is only important to know on what principle it is compiled.
Rarely does production operate without steel, so understanding its grades is extremely important
The alloy is designated by letters and numbers, which can be used to accurately determine which chemical elements it contains and in what quantity. Knowing this, as well as how each of these elements can affect the finished alloy, it is possible to determine with a high degree of probability exactly what technical characteristics are characteristic of a particular grade of steel.
Undesirable impurities
The sulfur contained in the melt, mainly in the form of FeS, does quite a lot of harm to it. This element imparts red brittleness to steel - high-temperature brittleness, which manifests itself, for example, during forging. It also increases the metal's tendency to abrasion, reduces its resistance to fatigue, and negatively affects corrosion resistance. The negative impact of sulfur can be reduced by adding manganese to the alloying composition, which binds this element. Also, to neutralize sulfur, lanthanum, cesium, and neodymium are introduced into the metal.
Another significant harmful impurity is phosphorus. It reduces the toughness of steel at low temperatures - this property is called cold brittleness. However, the presence of phosphorus in the alloy promotes better chip separation, and this improves the machinability of the material.
Nitrogen and oxygen are usually present in the metal in a chemically bound form and enter the steel as part of the ore, fuel, or deoxidizer added to the charge. Their presence reduces the viscosity and ductility of the metal, they contribute to the formation of weak formations in the alloy - oxides, nitrides, which become stress concentrators in the metal mass and seriously impair the endurance and viscosity of the material, making it susceptible to brittle fracture. Nitrogen, in addition, can lead to strain aging of steel.
Hydrogen is a very undesirable impurity. The result of its presence in the metal in quantities exceeding the permissible:
- fragility of steel;
- formation of flakes - defects in the form of round or oval silver-white cracks;
- decrease in viscosity;
- deterioration or complete loss of plasticity.
A properly composed alloying composition and quality control of the alloy steel produced at all stages of its production make it possible to reduce the negative impact of harmful impurities to a minimum.
Designations by type
Structural steel of ordinary quality and not containing alloying elements according to the requirements of GOST 380-94 is designated by the letters “St” and, depending on the composition, by numbers from 0 to 6. Metal of higher quality receives a lower number. The letter “G” indicates a high proportion of Mn in the metal. The metal group is indicated before the brand itself.
The greater the presence of carbon in the metal and the strength of the steel, the greater the indicated figure. To indicate a subcategory of steel, a number is added to the grade sign at the end of a certain category; the first of them, as a rule, is not indicated. After indicating the type and grade number, the degree of deoxidation is indicated. For example, St1kp2 means:
- St – carbon of normal quality
- Marks are the first
- kp – boiling
- 2 – second category
Sometimes the markings are longer, for example, chemically resistant alloy steel 12Х18Н10Т. Explanation:
- The 12 at the beginning indicates the carbon content – 0.12%. In the absence of numbers, it is assumed that carbon is greater than 1%.
- X18 means that the chromium in the alloy is 18%
- H10 – 10% nickel
- T is titanium, the absence of a digital index means the mass fraction is less than 1%
Designations of alloying elements
In order to recognize the qualitative and quantitative composition from the markings, letter designations are used for alloying elements. Basically, Russian letters correspond to the names of elements, although there are exceptions, since there are elements that begin with the same letters. The table of alloying elements is as follows.
Designation of alloying elements in steels
| IN | Tungsten | B | Niobium |
| TO | Cobalt | E | Selenium |
| M | Molybdenum | R | Bor |
| N | Nickel | F | Vanadium |
| T | Titanium | C | Zirconium |
| X | Chromium | YU | Aluminum |
| G | Manganese | A | Nitrogen |
| D | Copper | WITH | Silicon |
As can be seen from the table, it contains two non-metals - silicon and nitrogen, but no carbon. The presence of carbon is implied in the composition of any steel, therefore the designation indicates only its content