general information
So, steel is an alloy of Fe + C, (C – no more than 2%) + other elements. Steel is divided into carbon and alloy, taking into account the chemical composition, and based on the application - structural and instrumental . Special steels with specific characteristics are also produced for use in aggressive environments; such steels include heat-, corrosion-, and acid-resistant steels.
The quality of steel is determined by the production method and the amount of bad impurities and is divided into ordinary, high-quality, enhanced and high quality.
Description of mechanical symbols
| Name | Description |
| Section | Section |
| sT|s0.2 | Yield strength or proportional limit with tolerance for permanent deformation - 0.2% |
| σB | Short-term strength limit |
| d5 | Elongation after break |
| d4 | Elongation after break |
| y | Relative narrowing |
| kJ/m2 | Impact strength |
Chemical composition of ordinary quality steels
There is a typification based on the nature of solidification in the mold and the geometric shape of the ingot (mold shape). There are calm, semi-calm and boiling .
Carbon steel
Carbon steel is smelted without the addition of any alloying elements and is of ordinary and high quality.
Standard quality steels are usually divided into the following groups:
- group A - ensured by mechanical properties. Products made from steels of this group are used for subsequent welding, forging, etc. Moreover, the declared fur. properties may change. (St3, St5kp.).
- group B – steel is provided according to chemical requirements. composition. It is used for the manufacture of parts, during processing of which the mechanical characteristics determined by the composition may change.
Steel from group B is divided into 2 categories:
- 1st - the content of C, Si, Mn was established; limited content: S, P, N, As,
- 2nd - the amount of Cr, Ni, Cu is additionally limited.
- group B - ensured by mechanical characteristics and content of chemical elements. Used in the production of welded parts.
Divided into six categories.
Group B is designated as follows: steel grade, degree of deoxidation, category number. They have the same composition as steel of category 2, group B.
Steel marking
Considering, as an example, the marking of steel St5ps (structural carbon steel of ordinary quality).
- this steel belongs to group A (since the category is indicated before the letters St (VSt1, VSt2), and only group A is not indicated).
- number 5 - determines the conditional number of the brand based on the chemical. composition and mechanical properties.
- ps is the degree of deoxidation.
If after the number defining the steel grade there is a letter G, then the steel contains a suspended amount of manganese. (St25G2S)
Degrees of steel deoxidation
There are 3 degrees of steel deoxidation.
The deoxidation process allows you to restore iron oxide and bind dissolved oxygen, thus reducing its harmful effects.
Boiling steel
Boiling steel is not completely deoxidized. During casting into molds, it boils due to the abundant release of gas, so it is the most contaminated with gases and inhomogeneous. That is, the mechanical properties of the ingot may differ , since the distribution of chemical elements throughout the ingot is not uniform. The head part of the ingot contains the largest amount of carbon and various bad impurities (such as sulfur or phosphorus), which requires removal of part of the ingot (5% of the total mass).
The accumulation of sulfur in certain areas can cause a crystallization crack to appear along the seam. In these areas, steel is less resistant to aging and is most brittle at sub-zero temperatures. The silicon content in boiling steel does not exceed 0.07%.
So, we can say about boiling steel that it is quite brittle, has poor weldability and is most susceptible to corrosion. Therefore, in order to improve the characteristics of steel, it is deoxidized with silicon (0.12-0.3%), aluminum (up to 0.1%) or manganese (deoxidation is also possible with other chemical elements that dynamically react with oxygen). Boiling steel is quite brittle, has poor weldability and is most susceptible to corrosion.
The deoxidation process allows you to restore iron oxide and bind dissolved oxygen, reduce its harmful effects, while maintaining a high temperature of the steel for a long time, which promotes maximum gas and slag removal, as well as obtaining a micro-grained structure due to the formation of crystallization areas. Due to the formation of these foci, the quality of steel improves.
Liquation is the formation of a heterogeneous chemical structure of steel that occurs at the moment of crystallization. I distinguish two types of liquation: intracrystalline and dendritic. This phenomenon was first discovered by Russian metallurgists N.V. Kalakutsky and A.S. Lavrov in 1866.
Calm steel
The steel obtained as a result of deoxidation is called calm steel. The silicon content in smooth steel is at least 0.12%, and the presence of non-metallic inclusions and slag is minimal.
Mild steel ingots have a dense, homogeneous structure, and, accordingly, improved mechanical properties. Mild steel is excellent for welding and also has better resistance to impact loads. Is more homogeneous. It is suitable for the construction of supporting metal structures (due to its resistance to brittle fracture) that are subject to heavy loads.
Mild steel is excellent for welding and also has better impact resistance and is more uniform.
Semi-quiet steel
Semi-quiet steel is intermediate in quality indicators.
It is semi-deoxidized and crystallizes without boiling, releasing a sufficient amount of gas and having fewer bubbles than boiling steel. Therefore, semi-quiet steel has average quality indicators (as close as possible to calm steel), and sometimes replaces calm steel.
The cost of semi-quiet steel is slightly lower than that of calm steel, and the yield of high-quality rolled products from such ingots is 8-10% better.
The quality indicators of semi-quiet became closer to calm.
Semi-quiet steel hardens without boiling, but with the release of a large amount of gas. In such an ingot the content of bubbles is less than in a boiling ingot, but more than in a calm one.
Since the production of boiling steel is cheaper than calm and semi-quiet steel, it is widely used for the production of the least critical rolled metal products, such as wire rod, strip, angle, and hardware.
Designations
| Name | Meaning |
| Designation GOST Cyrillic | St3kp |
| Designation GOST Latin | Ct3kp |
| Translit | St3kp |
| By chemical elements | 3 |
| Name | Meaning |
| Designation GOST Cyrillic | VSt3kp |
| Designation GOST Latin | BCt3kp |
| Translit | VSt3kp |
| By chemical elements | 3 |
Boiling steel
Boiling steel, unlike calm steel, is incompletely deoxidized steel. During pouring in. During the crystallization process of the ingot, this steel “boils” in the mold. “Boiling” of the metal in the mold is caused by the abundant release of gases due to the reaction between carbon and iron or manganese oxide with the formation of carbon monoxide. The gases released during the solidification of a boiling steel ingot contain 80-90% CO, 1-2% CO2 and small amounts of hydrogen, nitrogen and methane.
The structure of the ingot is significantly influenced by the duration and intensity of steel boiling in the mold, which in turn depend on the rate of formation and release of carbon monoxide.
The structure of a boiling steel ingot is characterized by the following five zones:
1) outer bubble-free dense crust, consisting of equiaxed crystals;
2) a zone of honeycomb bubbles, which have an elongated shape and are located in the direction from the dense crust to the center of the ingot (the length of the honeycomb bubbles is 30-70 mm);
3) dense zone between cellular and secondary bubbles;
4) zone of secondary (deep) bubbles;
5) zone of central gas bubbles (ingot core).
The quality of a boiling steel ingot is determined by its height, as well as the quality and thickness of the outer crust. An ingot is considered to be of high quality if it has minimal growth, and its outer crust is dense and its thickness is sufficient for subsequent heating and rolling of the ingot without opening the honeycomb bubbles.
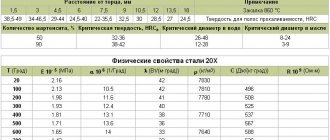
Mechanical characteristics
| Section, mm | sТ|s0.2, MPa | σB, MPa | d5, % | d4 | y, % | kJ/m2, kJ/m2 | Brinell hardness, MPa |
| Electric welded straight-seam pipes (Dy=10-530 mm) as delivered. The mechanical properties of the base metal are indicated | |||||||
| — | ≥225 | ≥372 | ≥22 | — | — | — | — |
| Forgings. Normalization | |||||||
| 100-300 | ≥175 | ≥355 | ≥24 | — | ≥50 | ≥590 | 101-143 |
| 100 | ≥175 | ≥355 | ≥28 | — | ≥55 | ≥640 | 101-143 |
| 100-300 | ≥195 | ≥390 | ≥23 | — | ≥50 | ≥540 | 111-156 |
| 100 | ≥195 | ≥390 | ≥26 | — | ≥55 | ≥590 | 111-156 |
| Hot-rolled thick sheets, sections and shapes in delivery condition | |||||||
| ≤20 | ≥235 | 360-460 | ≥27 | — | — | — | — |
| 20-40 | ≥225 | 360-460 | ≥26 | — | — | — | — |
| 40-100 | ≥215 | 360-460 | ≥24 | — | — | — | — |
| 100 | ≥195 | 360-460 | ≥24 | — | — | — | — |
| Hot-rolled thin sheets in delivery condition. Strength group OK360V | |||||||
| ≤2 | ≥235 | 360-530 | — | ≥20 | — | — | — |
| 2-3.9 | ≥235 | 360-530 | — | ≥22 | — | — | — |
| Cold-rolled thin sheets in delivery condition. Strength group OK360V | |||||||
| ≤2 | ≥235 | 360-530 | — | ≥22 | — | — | — |
| 2-3.9 | ≥235 | 360-530 | — | ≥24 | — | — | — |
| Sheets 12 mm thick as delivered (cross-sectional samples) | |||||||
| — | ≥205 | ≥385 | ≥37 | — | ≥60 | — | — |
| — | ≥190 | ≥370 | ≥27 | — | ≥59 | — | — |
| — | ≥175 | ≥430 | ≥21 | — | ≥51 | — | — |
| — | ≥160 | ≥450 | ≥23 | — | ≥49 | — | — |
| — | ≥150 | ≥395 | ≥35 | — | ≥62 | — | — |
The concept of boiling, calm and semi-quiet steel
All steel can be divided into three groups (boiling, calm and semi-calm).
Boiling steel, usually smelted in open-hearth furnaces and oxygen converters. This steel is not deoxidized before release in the furnace and therefore it contains increased amounts of dissolved oxygen, which did not have time to react with the carbon of the metal and turn into a reducing gas for iron and its oxides in the metal and slag - . The occurrence of the chemical reaction [C]+ [O]= is facilitated by an increase in temperature and the process of crystallization of the metal. The metal in the mold seems to “boil” due to gas evolution.
Deoxidation of steel occurs rapidly only due to the carbon of the metal. Some of the gases dissolved in the steel do not have time to escape into the atmosphere and the structure of the ingot becomes bubbly, i.e. contains discontinuities.
When rolling, the metal structure is compacted , because the non-oxidized internal and external surfaces of the bubbles are welded to the metal under rolling pressure. Boiling steel has high ductility because... for its deoxidation, special materials are not used - deoxidizers (for example, ferroalloys), the production and use of which is described in section 6.8 of the textbook. Therefore, non-plastic, insoluble non-metallic oxide and other inclusions that negatively affect the physical and mechanical properties of steel do not form in steel.
Calm steel is obtained only after complete deoxidation of the metal in a furnace or ladle before casting with the help of ferroalloys and other deoxidizers (for example, aluminum). Metal oxygen is bound into oxides by deoxidizing agents. There is no free oxygen necessary for the main reaction of the steelmaking process to occur (deoxidation of the metal by carbon) and “boiling” of the steel in the mold does not occur .
The ingots solidify calmly and have a dense macrostructure. In terms of a number of physical and mechanical properties, calm steel is superior to boiling steel, but it is more expensive than boiling steel due to the cost of deoxidizers.
To find a consensus between the requirements of mechanical engineering and the economics of steelmaking, metallurgical technologists developed a method for producing semi-quiet steel . This steel is partially deoxidized before casting (in a furnace, ladle). To obtain high-quality semi-quiet steel, it is necessary to ensure such an oxygen concentration in it that the metal in the molds does not “boil”; but at a certain stage of solidification, characterized by an increase in the carbon content in the metal due to segregation, the process of formation of carbon monoxide bubbles begins in the non-solidifying part of the ingot. These bubbles remain in the body of the ingot and the shrinkage cavity concentrated in the head part of the ingot, characteristic of the structure of ingots at quiet stages, is no longer formed. To obtain semi-quiet steel ingots, it is necessary to ensure the optimal silicon content in it - (0.08.0.15)%.
Semi-quiet steel, in comparison with boiling steel, has better mechanical properties, greater homogeneity of structure, increased cold resistance and resistance to “ aging ”. All this allows, in some cases, the use of semi-quiet steel as a substitute for calm steel. The yield of suitable rolled products from semi-damped steel ingots is (3.8)% higher than from mild steel ingots.
The production of semi-quenched steel requires more precise deoxidation technology. As mentioned above, semi-quiet steel is poured into through molds that are widened towards the bottom. If it is necessary to quickly interrupt the “boiling” of steel in the mold, technologies for mechanically clogging the metal in the mold are used, as when casting boiling steel.
An important task of casting is to protect the surface of the ingot from the formation of steel-melting films on it, as a result of steel splashes hitting the inner surface of the mold.
In addition to such technological methods as lubricating the internal surface of the mold, giving curved shapes to the contact surfaces of the pallets, regulating the speed of filling the mold with liquid steel during its casting, at enterprises with sheet rolling production, when preparing the mold for casting from above, a so-called “ cuff” » - a hard, unannealed thin cold-rolled sheet rolled into a pipe. When it unfolds, it covers the walls of the mold along the entire inner surface and is the first to come into contact with the stream of liquid steel and melt at the same time, acting as a fuse for the surface of the ingot from the formation of film on it.
An equally important task of steel casting is to protect it from oxidation and saturation with gases. To prevent the occurrence of such defects in any casting methods, the following technological methods are used:
●pouring in an atmosphere of inert gas - argon; it is expensive, but the most effective for this type of protection of poured steel; it can be used in the production of expensive, alloy steel grades; either the entire mold with steel is placed in a chamber filled with argon, or a stream of argon is applied to a stream of liquid steel in such a way that it, like a ring (cylinder), surrounds the stream of steel, and then fills the internal cavity of the mold;
●pouring using a frame made of timber; A wooden frame (frame) is placed on the bottom of the mold or its tray before steel casting begins; when the mold is filled with steel, the wooden structure floats up and gradually burns out; Gases formed during wood combustion protect the surface of the ingot from oxidation;
What is the name of heat treatment consisting of hardening and high tempering?
A) Normalization
B) Improvement
C) Spheroidization
D) Full hardening
What is the name of the treatment that consists of holding a hardened alloy for a long time at room temperature or at high heat?
A) Recrystallization
B) Normalization
C) High holiday
D) Aging
What is the name of the treatment that involves saturating the steel surface with carbon?
A) Cementation
B) Normalization
C) Improvement
D) Cyanidation
What is a carburizer?
A) A substance that serves as a source of carbon during cementation.
B) Carbides of alloying elements.
C) Device for producing air-fuel medium
D) A mixture of carbon dioxide salts.
Test task No. 5
To the topic “Classification and marking of steels and alloys”
Which of the steels given in the answers is hypereutectoid?
A) Art. 1 kp
B) U 10A
C) 10 ps
D) A 11
Which of the signs can characterize boiling steel?
A) Low silicon content
B) High ductility of the casting
C) Low ductility
D) Low manganese content
What kind of steel is called boiling steel (steel 3kp)?
A) Steel with increased strength
B) Steel brought to boiling point.
C) Steel deoxidized with manganese, silicon and aluminum
D) Steel deoxidized with manganese only
What quality category does Steel 6sp belong to?
A) To high-quality steels
B) To especially high-quality steels
C) To high-quality steels
D) To steels of ordinary quality
What quality category does 0.8 kp steel belong to?
A) To steels of ordinary quality
B) To high-quality steels
C) To high-quality steels
D) For particularly high-quality steels
What steels are called automatic?
A) Steels intended for the manufacture of critical springs operating in automatic devices.
B) Steels operating for a long time under cyclic alternating loading
C) Steels with improved machinability, having a high sulfur content or additionally alloyed with lead, selenium or calcium.
D) Tool steels intended for the manufacture of metal-cutting tools operating on automatic machines
Which group of materials does grade A 20 alloy belong to?
A) For carbon tool steels
B) To high-quality carbon structural steels
C) For steels with high machinability
D) To steels of ordinary quality
Which group of materials does AC40 alloy belong to? What is its chemical composition?
A) High quality structural steel. Contains about 0.4% carbon and about 1% flint.
B) Anti-friction cast iron. The chemical composition is not shown on the stamp.
C) Structural steel alloyed with nitrogen and silicon. Contains about 0.4% carbon.
D) Automatic steel. Contains about 0.4% carbon, increased amount of sulfur, alloyed with lead
What metals are called heat-resistant?
A) Metals that can resist frequent alternating heating and cooling.
B) Metals that can resist the corrosive effects of gas at high temperatures.
C) Metals capable of maintaining the martensite structure at high temperatures.
D) Metals that can resist deformation and destruction for a long time at elevated temperatures.
What metals are called heat-resistant?
A) Metals capable of maintaining the martensite structure at high temperatures.
B) Metals that can resist the corrosive effects of gas at high temperatures.
C) Metals that can resist deformation and destruction for a long time at elevated temperatures.
D) Metals capable of resisting frequent alternating heating and cooling.
Test task No. 6
On the topic “Non-ferrous metals and alloys”
Steel grades - table with markings and interpretation
Any specialist who deals with metal is familiar with the concept of “steel grade”. Deciphering the markings of steel alloys makes it possible to get an idea of their chemical composition and physical characteristics. Understanding this marking, despite its apparent complexity, is quite simple - it is only important to know on what principle it is compiled.
Rarely does production operate without steel, so understanding its grades is extremely important
The alloy is designated by letters and numbers, which can be used to accurately determine which chemical elements it contains and in what quantity. Knowing this, as well as how each of these elements can affect the finished alloy, it is possible to determine with a high degree of probability exactly what technical characteristics are characteristic of a particular grade of steel.
Types of steels and features of their markings
Steel is an alloy of iron and carbon, the content of which is no more than 2.14%. Carbon gives the alloy hardness, but if it is in excess, the metal becomes too brittle.
One of the most important parameters by which steels are divided into different classes is the chemical composition. Among the steels according to this criterion, alloyed and carbon steels are distinguished, the latter are divided into low-carbon (carbon up to 0.25%), medium-carbon (0.25–0.6%) and high-carbon (they contain more than 0.6% carbon).
By including alloying elements in the steel, it can be given the required characteristics.
It is in this way that, by combining the type and quantitative content of additives, grades with improved mechanical properties, corrosion resistance, magnetic and electrical characteristics are obtained.
Of course, it is possible to improve the characteristics of steels using heat treatment, but alloying additives make it possible to do this more efficiently.
Based on the quantitative composition of alloying elements, low-, medium- and high-alloy alloys are distinguished. In the first alloying elements there is no more than 2.5%, in medium alloyed elements - 2.5–10%, in highly alloyed ones - more than 10%.
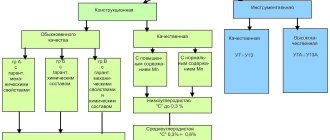
Steels are classified according to their purpose. Thus, there are instrumental and structural types, grades distinguished by special physical properties.
Tool types are used for the production of stamping, measuring, and cutting tools, structural ones - for the production of products used in construction and mechanical engineering.
Alloys with special physical properties (also called precision) are used to make products that must have special characteristics (magnetic, strength, etc.).
Classification of steels by purpose
Steels are also contrasted with each other based on their special chemical properties. Alloys of this group include stainless, scale-resistant, heat-resistant, etc. Typically, stainless steels can be corrosion-resistant and stainless steel for food - these are different categories.
In addition to useful elements, steel also contains harmful impurities, the main ones of which are sulfur and phosphorus. It also contains gases in an unbound state (oxygen and nitrogen), which negatively affects its characteristics.
If we consider the main harmful impurities, phosphorus increases the brittleness of the alloy, which is especially pronounced at low temperatures (the so-called cold brittleness), and sulfur causes cracks in metal heated to high temperatures (red brittleness).
Phosphorus, among other things, significantly reduces the ductility of heated metal.
Based on the quantitative content of these two elements, steels are divided into ordinary quality (no more than 0.06–0.07% sulfur and phosphorus), high-quality (up to 0.035%), high-quality (up to 0.025%) and especially high-quality (sulfur - up to 0.015%, phosphorus - up to 0.02%).
The marking of steels also indicates to what extent oxygen has been removed from their composition. According to the level of deoxidation, steels are divided into:
- calm type, designated by the letter combination “SP”;
- semi-calm - “PS”;
- boiling - “KP”.
What does the steel marking mean?
It has become quite easy to decipher the brand; you just need to have certain information. Structural steels of ordinary quality and not containing alloying elements are marked with the letter combination “St”.
By the number following the letters in the name of the brand, you can determine how much carbon is in such an alloy (calculated in tenths of a percent). The numbers may be followed by the letters “KP”: from them it becomes clear that this alloy has not completely gone through the deoxidation process in the furnace, and accordingly, it belongs to the boiling category.
If the brand name does not contain such letters, then the steel corresponds to the calm category.
Chemical composition of carbon structural steels of ordinary quality
Structural unalloyed steel, which belongs to the quality category, has two numbers in its designation; they are used to determine the average carbon content in it (calculated in hundredths of a percent).
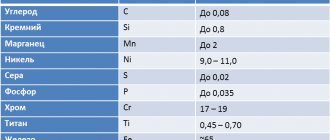
Before we begin to consider the grades of those steels that include alloying additives, you should understand how these additives are designated. Marking of alloy steels may include the following letter designations:
List of alloying additives used
Designation of steels with alloying elements
As mentioned above, the classification of steels with alloying elements includes several categories. The marking of alloy steels is compiled according to certain rules, knowledge of which allows one to quite simply determine the category of a particular alloy and the main area of its application.
In the initial part of the names of such brands there are numbers (two or one) indicating the carbon content. Two numbers indicate its average content in the alloy in hundredths of a percent, and one – in tenths. There are also steels that do not have numbers at the beginning of the brand name.
This means that the carbon content in these alloys is within 1%.
Example of alloy steel marking
The letters that can be seen behind the first digits of the brand name indicate what the alloy is made of.
The letters that give information about a particular element in its composition may or may not be followed by numbers.
If there is a number, then it determines (in whole percentages) the average content of the element indicated by the letter in the alloy, and if there is no number, it means that this element is contained in the range from 1 to 1.5%.
At the end of the marking of certain types of steel there may be the letter “A”. This suggests that this is high quality steel. These grades may include carbon steels and alloys with alloying additives in their composition. According to the classification, this category of steels includes those in which sulfur and phosphorus amount to no more than 0.03%.
Examples of marking steels of various types
Determining the grade of steel and assigning an alloy to a certain type is a task that should not cause any problems for a specialist. You don’t always have a table at hand that gives a breakdown of brand names, but the examples given below will help you figure it out.
elements in common steel grades (click to enlarge)
Structural steels that do not contain alloying elements are designated by the letter combination “St”. The numbers following are the carbon content, calculated in hundredths of a percent. Low-alloy structural steels are marked somewhat differently.
For example, 09G2S steel contains 0.09% carbon, and alloying additives (manganese, silicon, etc.) are contained within 2.5%. 10KhSND and 15KhSND, which are very similar in their markings, differ in different amounts of carbon, and the share of each alloying element in them is no more than 1%.
That is why there are no numbers after the letters indicating each alloying element in such an alloy.
20Х, 30Х, 40Х, etc. – this is how structural alloy steels are marked; the predominant alloying element in them is chromium.
The number at the beginning of such a mark is the carbon content in the alloy in question, calculated in hundredths of a percent.
The letter designation of each alloying element can be followed by a number, which is used to determine its quantitative content in the alloy. If it is not there, then the specified element in the steel contains no more than 1.5%.
You can consider an example of the designation of chromium-silicon-manganese steel 30KhGSA. According to the labeling, it consists of carbon (0.3%), manganese, silicon, and chromium. It contains 0.8–1.1% of each of these elements.
How to decipher steel markings?
To make deciphering the designations of different types of steels easy, you should know well what they are. Certain categories of steel have special markings. They are usually designated by certain letters, which allows you to immediately understand both the purpose of the metal in question and its approximate composition. Let's look at some of these brands and understand their designation.
Properties and purpose of structural alloy steels
Structural steels specially intended for the manufacture of bearings can be recognized by the letter “Ш”; this letter is placed at the very beginning of their marking.
After it in the brand name there is a letter designation of the corresponding alloying additives, as well as numbers by which the quantitative content of these additives is determined.
Thus, steel grades ShKh4 and ShKh15, in addition to iron and carbon, contain chromium in amounts of 0.4 and 1.5%, respectively.
The letter “K”, which appears after the first digits in the brand name, indicating the quantitative carbon content, denotes structural non-alloy steels used for the production of vessels and steam boilers operating under high pressure (20K, 22K, etc.).
High-quality alloy steels, which have improved casting properties, can be recognized by the letter “L” at the very end of the marking (35ХМЛ, 40ХЛ, etc.).
Deciphering the grades of construction steel can cause some difficulty if you do not know the specifics of the markings. Alloys of this category are designated by the letter “C”, which is placed at the very beginning. The numbers following it indicate the minimum yield strength. These brands also use additional letter designations:
- letter T – heat-strengthened rolled products;
- letter K – steel, characterized by increased corrosion resistance;
- letter D is an alloy characterized by a high copper content (S345T, S390K, etc.).
Unalloyed steels belonging to the tool category are designated by the letter “U”; it is affixed at the beginning of their marking. The number following this letter expresses the quantitative carbon content in the alloy in question.
Steels of this category can be high-quality and high-quality (they can be identified by the letter “A”, it is placed at the end of the brand name).
Their marking may contain the letter “G”, which means a high content of manganese (U7, U8, U8A, U8GA, etc.).
Tool steels containing alloying elements in their composition are marked similarly to alloyed structural steels (KhVG, 9KhVG, etc.).
Composition of alloy tool steels (%)
The marking of those steels that are included in the high-speed cutting category begins with the letter “P”, followed by numbers indicating the quantitative content of tungsten. Otherwise, brands of such alloys are named according to the standard principle: letters denoting the element, and, accordingly, numbers reflecting its quantitative content.
The designation of such steels does not indicate chromium, since its standard content in them is about 4%, as well as carbon, the amount of which is proportional to the vanadium content.
If the amount of vanadium exceeds 2.5%, then its letter designation and quantitative content are affixed at the very end of the marking (З9, Р18, Р6М5Ф3, etc.).
The influence of some additives on the properties of steel
Unalloyed steels belonging to the electrical category are marked in a special way (they are also often called pure technical iron). The low electrical resistance of such metals is ensured due to the fact that their composition is characterized by a minimum carbon content - less than 0.04%. There are no letters in the designation of grades of such steels, only numbers: 10880, 20880, etc.
The first digit indicates the classification by type of processing: hot-rolled or forged - 1, calibrated - 2. The second digit is associated with the category of the aging coefficient: 0 - non-standardized, 1 - standardized. The third digit indicates the group to which this steel belongs according to the standardized characteristic taken as the main one.
The value of the standardized characteristic itself is determined from the fourth and fifth digits.
The principles by which the designation of steel alloys is carried out were developed back in the Soviet period, but to this day they are successfully used not only in Russia, but also in the CIS countries. Having information about a particular grade of steel, you can not only determine its chemical composition, but also effectively select metals with the required characteristics.
Understanding this issue is important both for specialists who develop and design various metal structures, and for those who often work with various steels and manufacture parts from them for various purposes.
Boiling steel
Boiling steels (- 0 05 - 0 07% Si) are completely undeoxidized, and before solidification they contain an increased amount of FcO. When FeO solidifies in the mold, it reacts with the carbon in the metal to form CO, which bubbles up in the metal and gives the appearance of the metal boiling. A boiling steel ingot is characterized by a large number of gas bubbles, as a result of which there is practically no shrinkage cavity in it. If the walls of the bubbles are unoxidized, then during hot rolling the bubbles are welded. Boiling steels are cheaper, since waste during their production is minimal. Compared to mild and semi-mild steels, they are more prone to aging and cold brittleness and are less weldable. Nevertheless, boiling steels have high ductility and are easily drawn when cold. When marking, additionally indicate the checkpoint. [1]
Boiling steel containing 0.07% Si is obtained by incomplete deoxidation of the metal with manganese. It is characterized by a pronounced uneven distribution of sulfur and phosphorus throughout the thickness of the rolled product. Boiling steel is prone to aging in the cold zone and transition to a brittle state at subzero temperatures. Mild steels are obtained by deoxidation with manganese, aluminum and silicon and contain 0-12% Si; sulfur and phosphorus are distributed more evenly in them than in boiling steels. These steels are less prone to aging and less responsive to welding heat. Semi-quiet steel, in terms of its tendency to aging, occupies an intermediate position between boiling and calm steels. Ordinary quality steel is supplied without heat treatment in a hot-rolled state. Structures made from it are also not subjected to subsequent heat treatment. [2]
Boiling steel is more ductile; it can be welded and stamped, so it is used for deep drawing parts, welded pipes and other products. Boiling steel is cheaper than calm steel, since ingots from it can be obtained without profit due to the absence of a concentrated shrinkage cavity. [4]
During the smelting process, boiling steel is not treated with chemicals that can combine with gases and other harmful impurities and form slags that easily float to the surface of the bath, so when the metal solidifies, gas bubbles remain in the ingot. If the megall around the bubble is not oxidized from the surface, then during subsequent pressure treatment (rolling, forging) the bubbles are welded and the continuity of the metal is not broken. Otherwise, defective places remain in the metal, violating the continuity and continuity of the product, weakening it and being places of stress concentration during the operation of a structure made of this steel. [5]
Boiling steel is produced according to the 2nd category - VStZkp2, semi-quiet - according to the 6th category - VStZpsb, calm and semi-quiet with a high manganese content - according to the 5th category - VStZspb and VStZGpsb. [7]
Boiling steel is not completely deoxidized in the furnace. Its deoxidation continues in the mold during casting and solidification due to the interaction of FeO and carbon contained in the metal. The carbon monoxide formed during the reaction of FeO with FeCO is released from the steel, helping to remove nitrogen and hydrogen dissolved in the steel. Gases are rapidly released from the steel in the form of bubbles, causing it to boil. The boiling of the metal in the mold mixes the steel, equalizes its temperature in different places of the ingot, which reduces the formation of shrinkage defects. At the same time, this affects the appearance of chemical heterogeneity of the metal in different parts of the ingot. The process of gas evolution also occurs during the solidification of the ingot, so a large number of gas shells (bubbles) are formed in it, which are welded when the ingot is rolled. [8]
Standards
| Name | Code | Standards |
| Ribbons | B34 | GOST 19851-74 |
| Steel pipes and connecting parts for them | B62 | GOST 3262-75, GOST 8696-74, GOST 10704-91, GOST 10705-80, GOST 10706-76, GOST 10707-80, GOST 12132-66, TU 14-3-1160-83, TU 14-3-1428 -86, TU 14-3Р-56-2001, TU 1373-013-02949352-2003 |
| Ribbons | B24 | GOST 3560-73, GOST 6009-74, STP M309-74 |
| Classification, nomenclature and general norms | IN 20 | GOST 380-2005 |
| Long and shaped rolled products | B22 | GOST 5267.0-90, GOST 5422-73, GOST 5781-82, GOST 7511-73, GOST 8239-89, GOST 8240-97, GOST 8278-83, GOST 8281-80, GOST 8282-83, GOST 8283-93, GOST 8509-93, GOST 8510-86, GOST 9234-74, GOST 10551-75, GOST 11474-76, GOST 12492.1-90, GOST 12492.13-90, GOST 12492.14-90, GOST 19240-73, GOST 19425-74 , GOST 19771-93, GOST 19772-93, GOST 25577-83, GOST 30565-98, GOST 535-2005, GOST 30136-95, GOST 2590-2006, GOST 2591-2006, GOST 2879-2006, OST 5.9086-85, GOST OST 5.9087-84, TU 14-2-341-78, TU 14-2-949-91, TU 14-1-5283-94, TU 14-1-5254-94 |
| Rails. Overlays. Linings. Crutches | B42 | GOST 5812-82, GOST 8142-89, GOST 16277-93 |
| Sheets and strips | B23 | GOST 82-70, GOST 8568-77, GOST 14637-89, GOST 14918-80, GOST 16523-97, GOST 19903-74, GOST 16523-89, GOST 16523-70, GOST 103-2006, GOST 19903-90, OST 5.9094-85, TU 36.26.11-5-89, TU 14-1-3023-80, STP M324-90 |
| Metal forming. Forgings | B03 | GOST 8479-70, OST 5R.9125-84, ST TsKBA 010-2004 |
| Blanks. Blanks. Slabs | B31 | OST 3-1686-90, TU 14-1-4944-90 |
| Welding and cutting of metals. Soldering, riveting | B05 | OST 36-58-81 |
| Sheets and strips | B33 | TU 14-1-3579-83, TU 14-1-4431-88 |
| Long and shaped rolled products | B32 | TU 14-11-245-88, TU 14-1-1271-75, TU 14-136-367-2008 |