Aluminum is perhaps the most common metal in everyday life. In almost every home there are many small objects made of aluminum, this includes various kinds of key rings, gift imitation knives, metal coatings for flash drives, etc. - the list can be continued almost endlessly.
It is quite natural for any man to want to make his small accessories unique. For metal objects, the idea of improvement comes naturally: engraving. To engrave products, you can use a laser engraver. However, this method is not suitable for aluminum.
The melting point of the metal is 600 degrees Celsius. It is difficult to find an engraver for engraving metal with such a melting point, but it is possible. But we have to take into account the oxide film that covers the surface of the metal when it comes into contact with oxygen. And the melting temperature of this film is about 2000 degrees Celsius. This means that the most common method of applying engravings and inscriptions, thermal, is not suitable. It is in such situations that it is worth remembering another method: etching. Note that etching can be both artistic (to create drawings and engravings on the surface of the metal) and technical, with the aim of clearing the metal surface of the oxide film and making the metal suitable for further processing.
Aluminum etching can be done in two ways:
- Electrolytic method.
- Chemical method.
We will analyze each of the methods, as well as their implementation at home, in detail.
Types of etching
There are two main types of etching of metals in general and aluminum in particular: chemical and galvanic. The last method is just artistic.
For chemical use: the product is placed in a container into which a solution of hydrochloric or sulfuric acid has been previously poured. In the same way, the aluminum workpiece is etched with an alkali, for example caustic soda.
And galvanic (otherwise known as electrolytic or electrochemical) occurs thanks to an electric battery. The process itself is carried out in a special bath, where there is an anode and a cathode.
Next, each of the aluminum etching methods will be discussed in more detail. We will also find out which method is the safest at home.
Dear friends and colleagues!
Congratulations on our professional holiday - Chemist's Day!
Chemistry is a great science that captivates you completely and forever. Requires attention, careful and creative attitude.
Firmly remember and follow the commandments of the electroplating technologist:
1. Keep the electrolyte clean and tidy, just like your body.
2. I came to the regime - don’t flutter: the best is the enemy of the good.
3. The coating is not going well - look at the root, change the skimming agent!
5. Honor regulatory documents as sacred scripture.
6. If you want to live without problems, convert the designer to your faith.
And let your love for chemistry be mutual!
Koroleva Galina Vladimirovna
Acid etching of aluminum
Due to the fact that very strong acids are used in this process, it is first of all necessary to take increased precautions when working with them. The operator must wear gloves, a mask, and an apron. It is important that the room where the process itself takes place is well ventilated. Without certain skills and without certain protective equipment, it is not recommended to work with acids.
As noted above, the aluminum product is placed in a container with acid. The most common reagents used for chemical etching of aluminum with acid are hydrochloric or sulfuric acid. When they interact with metal, hydrogen is released. Externally, it looks like this: the surface of the product is covered with small bubbles. But, in principle, this can be prevented if you add a special ingredient to the container in advance. This way the metal will be protected from bubbles by a thin film.
A very important point: all operations for etching an aluminum product with acid must be performed intensively so that the surface of the metal itself remains intact.
The described method is recommended to be carried out in containers made of wood or concrete. In this case, its inner surface should be lined with acid-resistant tiles so that the walls of the container do not corrode.
This method is not used very often in practice.
↑ How to use the recipe?
All this must be mixed before use in glass or plastic containers. The amount of ingredients can be changed proportionally, and more citric acid can be used
.
Etching time about 20 minutes
at room temperature, depends on the area of the board. Increasing the temperature does not lead to a significant increase in activity, so I believe that heating is not necessary
It is important to stir the etching solution to access fresh solution and wash away reaction products
The solution according to this recipe does not corrode hands and clothes
and does not stain the sink. Initially, the solution is transparent, but as it is used, it acquires a “sea wave”, greenish-bluish color.
Photo in progress, sent to Datagor Beso
(Minsk): “Indeed, it poisons quickly, poisons cleanly, and, most importantly, poisons cheaper than with ferric chloride.”
To correct LUT imperfections, a permanent marker, paint marker or nail polish is suitable. The solution is not stored, it is always better to etch in a freshly prepared mixture
.
My version of pickling in a bucket of some kind of food. The solution is used very economically.
They also offer an option on the Internet that involves replacing citric acid with 70% acetic acid. I believe that this can only be done as a last resort, because we end up with a stink and working in a more dangerous environment.
Aluminum etching with alkali
Most often, this method uses an aqueous solution of caustic soda (an option with or without additives is possible).
And it is used to clean the surface of an aluminum product from oxide or unnecessary lubricant and obtain a smoother (matte or glossy) surface.
Why is it necessary to clean so thoroughly? To ensure that the finished product (for example, decorative architectural elements, signs) has an ideal surface. This method is also used for deep engraving.
The method of etching aluminum with alkali, on the one hand, is quite inexpensive, but it is very labor-intensive.
Cold technology
To anodize aluminum you need:
- power supply 12 V (battery, stabilizer);
- aluminum wires;
- rheostat;
- ammeter;
- containers for solutions.
First, the preparatory work described above is carried out. Then the parts need to be secured. It should not be forgotten that a film does not form under the fastening element. And suspended workpieces, when lowered into the container, should not touch the walls and bottom.
The anode is connected to the parts from the power source, and the cathode is connected to the capacitance. The current density is selected within the range of 1.6-4 A/dm2. Recommended values 2-2.2 A/dm2. At small values, the process will proceed more slowly, and at large values, a breakdown of the circuit may occur and the coating will begin to collapse.
It is not recommended that the electrolyte temperature rise above 5°C. When anodizing, the electrolyte does not heat up evenly. It is warmer in the center than in the corners of the container, so constant stirring is necessary.
The duration of anodizing using the cold method is about half an hour for small elements. For large parts, the duration may be 60-90 minutes. The end of the process is indicated by a changed color on the surface of the aluminum product. After disconnecting the wires, the part is washed.
Features of this method
The solutions used contain from four to ten percent sodium. The temperature when etching with alkali is approximately 40-90 degrees Celsius.
If necessary, a moisturizer or special additive is used to obtain a light foamy coating on the workpiece.
The average temperature at the height of the process is sixty degrees. It is at these thermal parameters that high-quality surface cleaning occurs.
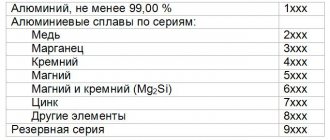
The optimal purity of aluminum is 99.5%, and the concentration of caustic soda solution is 10, 15, or 20%.
Thus, during the reaction, aluminum dissolves in sodium hydroxide, releasing hydrogen. As a result, a composite aluminate is formed, and it exists only in an alkali solution.
↑ Rolling photoresist
Next, we measure out the photoresist for the body and buttons.
Rice. 3. Film photoresist.
We measure a little with a margin around the edges so that it is convenient to roll. Film photoresist consists of 4 layers: the bottom (it is matte) - polyethylene, then a thin layer of glue, then, in fact, the photoresist itself, and on top there is a glossy protective layer (lavsan). Carefully pry up the matte layer with a needle or scalpel, tear off a strip 5-8 millimeters wide and glue it to the body. It is easier to roll the photoresist along the length of the body.
Yes! One more nuance. It is better to heat the housing over gas to a temperature of approximately 40 degrees. Then the photoresist sticks better. Gradually tearing off the base, we roll the photoresist onto the surface with a hard photo roller, or, at worst, with your finger. We cut off the protruding edges of the photoresist with a file to the body or a sharp knife.
Make sure that no dust particles or air bubbles get under the photoresist. In this place, ferric chloride may get in and there will be a problem. If air bubbles do occur, you can carefully pierce them with a sharp needle and roll them firmly with a photo roller. We do not remove the top protective layer yet, because the photomask may stick to the photoresist (there have been cases).
Rice. 4. Rolled photoresist.
Further processes occurring during etching with alkali
During this process, the amount of caustic soda gradually becomes less. And thus the speed of the process itself decreases, but the viscosity increases.
Provided that no sodium hydroxide was added to the container at all, the reaction can slow down very much. But eventually the brownish or clear aluminum etching solution turns white.
And from this moment on, the speed of the process increases.
As a result of the reaction, aluminum oxide hydrate precipitates, which looks like a suspension. Caustic soda is also released, which is also necessary for the etching process to continue.
↑ Making a photo template
Next, use any convenient program to prepare a photo template and print it on transparent film for printers. When printing, we indicate the maximum contrast and minimum brightness, but here you have to try. I have an Epson RX610. The settings are as follows: print quality “Best Photo”, “Shades of Gray”, paper type “Epson Matte”, brightness: -25, contrast +25. Photoresist is negative! That is, where there is no paint on the template, the photoresist will glow and will not wash off during development! Be careful.
Rice. 5. Photo template. I use film sparingly. Therefore, I print different projects on one sheet while there is space left.
Results with the method under consideration
It has been experimentally established that a solution of caustic soda, when used intensively during the etching process, begins to “absorb” aluminum. And this happens until the amount of caustic soda decreases to one-fourth of the original volume. And after this, the process will continue with free caustic soda, fluctuating in its quantity. And this, in turn, depends on temperature, frequency of use and intensity of stops (pauses).
In this case, the hydrate will slowly settle into sediment or form crystals at the bottom and/or sides of the container. The resulting hydrate will be quite dense and will not be easy to remove. Sometimes it tries to settle right on the surface of the heating coils.
There is another important point regarding the aluminum content. When etching products made of this metal in caustic soda, it is necessary to strictly observe the ratio of the amount of aluminum and soda. Because the more aluminum there is, the slower the process itself will occur. From a practical point of view, it becomes clear that it is necessary to constantly increase the amount of caustic soda as the amount of aluminum in the container increases.
Thus, the process of etching aluminum with alkali can be continued continuously. And losses of caustic soda will occur only due to its entrainment with steam.
This method is really applicable from a practical point of view. But there are several nuances that should not be forgotten: remove the hardened hydrate sediment from time to time; clean the filter; remember that the container in which the process is carried out, with constant use, can last no more than two years.
Otherwise, no complications regarding the use of this method were identified.
In total, after chemical etching of an aluminum workpiece, it is necessary to thoroughly rinse its surface, neutralize and lighten it with a 15-20% solution of nitric acid. This process is called pickling.
Maintaining the stability of the E6 bath
To obtain particularly matte surfaces, a high aluminum content is required, which can reach 200 g/l. With such a high aluminum content, special attention to bath stability is required [1].
Stable black bathtub E6
Just one glance is enough to determine whether the E6 bath is still stable. If it has a rich black color (Figure 1), then you can be sure that the bath is sufficiently stable. Of course, this visual assessment must always be confirmed by regular chemical analysis of the bath composition.
Figure 1 – E6 bath of rich black color in a stable state [1]
Gray bathtub E6 - what's going on?
If the E6 bath has turned gray instead of rich black, then you need to react immediately. In this case, the process of destabilization has already begun. During this process, white, poorly soluble particles of aluminum hydroxide fall out of solution. If their proportion in the bath continues to increase, then the bath becomes increasingly gray, that is, the black color changes to dark gray, then medium gray, then light gray, until it becomes completely white. In the light gray and white condition, the bathtub is considered lost and must be completely restored, which requires significant amounts of money, work and time. Therefore, the occurrence of such a condition should be avoided in every possible way.
Figure 2 – Dark gray bath E6 – beginning of destabilization [1]
How to save an unstable gray bathtub E6?
If the E6 alkaline bath has turned slightly gray, you must:
- Immediately perform a complete chemical analysis of the bath.
- The instability of the bath will be indicated by a decrease in the aluminum content, since part of the dissolved aluminum has already been released from the solution in the form of poorly soluble aluminum hydroxide.
- At the same time, the content of sodium hydroxide will increase, as it is released during destabilization of the solution.
Figure 3 – Chemical reaction of the destabilization process [1]
It would be a mistake not to add sodium hydroxide to the bath, although its content is most likely still within the specified range. Low alkalinity contributes to further destabilization of this bath [1]:
- A large amount of a special additive (for example Alfasatin) must be added to the gray bath for additional stabilization, for example twice as much as usual.
- It is recommended to increase the alkalinity of the bath by adding sodium hydroxide to further stabilize it.
These stabilization measures ensure that:
- no further precipitation of aluminum hydroxide occurred and
- The aluminum hydroxide remaining in the bath solution was gradually removed from the bath with the treated aluminum surfaces.
As a result, the bath should gradually return to a stable state (black). During this time, monitoring the bath chemistry is especially important. If the solution in the bath turns black again, the stabilization process can be considered successful.
Galvanic method
The second method of etching is galvanic. It is simpler and takes place much faster. And the result is a very high-quality surface of the product, clear contours of the design (with an artistic method, like a type of galvanic).
The peculiarity of this method is that it uses a source of electrical energy (4-5 V).
You will also need a bathtub of a size that will accommodate an aluminum product. The material from which the bath is made must be dielectric. The composition of the bath for etching aluminum is a solution of copper sulfate and table salt.
Before starting the process, the workpiece must be cleaned and degreased. Next, solder copper wire to the product with tin and lower it into a solution of caustic soda, and then into a solution of sulfuric acid. After 2 minutes, remove and rinse under hot water. It is prohibited to touch the product with your hands at this moment.
If some areas of the workpiece do not need to be etched, mastic is applied to them. After this, you can begin the process itself.
This method uses two so-called supports, which must be connected to the anode (positive charge) and cathode (negative charge) of the electricity source. It is important that these supports are positioned across the bathtub. A workpiece made of aluminum is attached to the support with the anode, and a workpiece made of another metal is attached to the second one.
All this is lowered into the bath and kept for a certain amount of time. After this, it is washed with turpentine and further processed by grinding and polishing.
↑ Poisoning in ferric chloride
We cover exposed areas of metal that do not need to be etched (for example, the ends) with colorless nail polish (you can steal it from your wife, like I did). Now we take a photo bath, pour in ferric chloride and throw the body and buttons there with the image DOWN.
Rice. 10. Etching.
The solution immediately begins to bubble. Aluminum displaces iron from the solution and it settles right there, at the site of etching. It should be removed with a soft, unnecessary toothbrush approximately once every 30 seconds. In this case, you need to be careful: chips of the photoresist may appear at the edges of the image. If this happens, immediately rinse, dry and correct the chip with a waterproof marker or the same nail polish. However, the varnish can corrode the photoresist, so be careful.
I etched for about 5 minutes. After etching, I get indentations about 0.5 mm deep. We remove the photoresist. When making printed circuit boards, photoresist can be removed with a solution of caustic soda (caustic soda) or slightly diluted “Mole” for cleaning sewer pipes. But this is not suitable for aluminum. It darkens on contact with caustic. If the etched recesses are deep, then you can remove the photoresist with an emery sponge and water, if not very deep, then you can throw it in a bowl with acetone or solvent No. 646 or 647 for 15-20 minutes.
Rice. 11. After etching and removal of photoresist.
↑ Idea
The milling machine ordered aluminum buttons. The case was purchased from a store. And then the question arose of how to make indelible inscriptions on the buttons and body. I tried to scratch it and fill it with paint. It came out completely “meh”! Can be engraved! So I don’t have a Dremel, but I can’t help but search through friends. Laziness, my friends, is the most powerful engine of progress. After some thought, I remembered that I had once accidentally dripped ferric chloride onto an aluminum radiator. While I wiped away the drop, there was a stain on the radiator and a small indentation. Yeah...
What if you make a stencil from photoresist and then etch it? The guinea pig was a piece of duralumin plate. Everything turned out great!