BASICS OF METALLURGICAL PRODUCTION OF IRON AND STEEL AND METAL SCIENCE
Chapter I
BASICS OF METALLURGICAL PRODUCTION OF IRON AND STEEL AND METAL SCIENCE
IRON AND STEEL PRODUCTION
CAST IRON PRODUCTION
Cast iron is produced in blast furnaces constructed using refractory materials. The production of cast iron is based on the method of melting raw materials, resulting in the reduction of iron and its saturation with carbon and other elements. The main raw materials for iron smelting are fuel, ores and fluxes.
In metallurgical production, coal coke, fuel oil and natural gas, which are substances of organic origin, are mainly used as fuel. They contain carbon, hydrogen, oxygen, nitrogen, sulfur, as well as moisture and mineral impurities that produce ash. Fuel is burned in melting and heating furnaces and devices.
In modern blast furnace production, coal coke is used, which, being a fuel, ensures heating of the furnace space to the required temperature and the occurrence of chemical reactions of the reduction of iron from iron ores during smelting.
Iron ores (iron ores) are minerals that are rocks or minerals mined from the depths of the earth. For the production of cast iron, red, brown magnetic and spar iron ores are used, as well as manganese ore, which contain a lot of ore substance (iron oxides) and little waste rock, i.e. minerals that are easily separated from iron ore when preparing it for smelting (roasting) and turn into slag during melting (they do not pollute the cast iron being smelted).
Harmful impurities in ore include sulfur, arsenic and phosphorus.
The main purpose of fluxes is to convert waste iron ore and coke ash into slag, located above liquid cast iron in a blast furnace, and give it sufficient fluidity.
The amount and types of fluxes depend on the amount and chemical composition of the waste iron ore, as well as the type of cast iron being smelted. Iron ore gangue usually contains silica, so limestone is typically used as a flux in the blast furnace process.
BLAST MELTING PRODUCTS
The products of the blast furnace are pig iron, slag and blast furnace gas. Cast iron is an alloy of iron and carbon (2.14–6.67%) with admixtures of manganese and silicon, as well as harmful impurities of phosphorus and sulfur.
Depending on the purpose, cast iron is smelted in three grades: conversion, foundry and special (ferroalloys).
Pig iron is intended for processing into steel. Its production accounts for more than 80% of the total volume of cast iron produced.
Foundry coke cast iron is intended for the production of iron castings of various profiles (shaped) by casting into molds.
Ferroalloys are intended for steel production. They differ from pig iron and foundry cast iron in their increased content of manganese or silicon and are called ferromanganese and ferrosilicon, respectively.
The slag generated during the smelting of cast iron is widely used in granular form in the production of cement, slag wool for thermal insulation, slag blocks and other building materials.
After cleaning from dust, blast furnace gases are used as gaseous fuel to heat the air going into blast furnaces (in air heaters-coupers), heat ladles, and also for other furnaces of a metallurgical plant.
DIRECT IRON RESTORATION
Direct reduction of iron is very promising due to its high purity. With this method, iron is reduced directly from the ore, as a result of which the metal is not contaminated with sulfur and other impurities from coke, as happens in the blast furnace process.
The resulting iron (reduction product) is a solid iron ore material in which most of it is in metallic form. From this iron steels with high mechanical, electrical and other properties are obtained.
When the reduction product contains 90–94% metallic iron, it is called metallized raw material; with a higher content, it is called sponge iron.
Reduced iron products are mainly used for smelting into steel in arc furnaces. In the direct reduction of iron, sinter and pellets are used as the initial iron ore raw material, and solid fuel or gas containing H2 and CO is used as a reducing agent. The two best known methods for reducing iron are reduction with gas in a thick layer and reduction with solid carbon.
STEEL PRODUCTION
Steel is an alloy of iron and carbon with a content of up to 2%, i.e. steel contains less carbon than cast iron. In addition to carbon, steel contains the same impurities as cast iron (manganese, silicon, sulfur, phosphorus, etc.), but in much smaller quantities.
The steel production process is based on methods of smelting pig iron or combining it with scrap metal and ore. When melting, fluxes and deoxidizers are introduced, and, if necessary, special alloying additives. At the same time, excess carbon, manganese and silicon, as well as harmful impurities (sulfur, phosphorus), are removed from the iron-carbon alloy by oxidation. Carbon combines with oxygen to form carbon monoxide CO, which burns and evaporates from the bath. Silicon, manganese and phosphorus form the oxides SiO2, MnO and P2O5, which float to form slag. The slag is then removed. Sulfur passes into the slag in the form of the CaS compound due to the addition of flux (lime) in the main smelting process.
Converter (oxygen-converter), open-hearth and electric arc (in electric arc furnaces) methods of steel smelting are widely used. The most highly productive methods of steel smelting are converter and electric arc. Steel production using these methods is increasingly increasing. A number of more expensive and less productive methods are known: vacuum arc remelting, vacuum induction remelting, electroslag remelting, remelting in electron beam and plasma furnaces. These methods are called remelting, since the processes involve remelting steel previously produced by converter, open-hearth or conventional electric arc methods. These methods make it possible to obtain steel of particularly high quality.
According to the conditions of steelmaking, the process is called acidic or basic. The process of steel smelting is called acid when the furnace (converter) lining, flux and slag consist of quartzite and quartz, i.e., substances containing silica SiO2 (acid oxide).
The main process of steel smelting is when the furnace (converter) lining, flux and slag consist of limestone, dolomite, dolomitized limestone or magnesite, the components of which are the main oxides of calcium CaO and magnesium MgO. Therefore, for example, steel smelted by the open-hearth method is called open-hearth acid or open-hearth basic steel, depending on the smelting conditions.
CONVERTER METHOD
The converter acid method of steel smelting is called the Bessemer method, the converter main method is called the Thomas method. With these smelting methods, steel is produced in converters by blowing cast iron from below with air, so it has many polluting gas inclusions (nitrogen, oxygen, hydrogen, etc.), as well as other impurities (sulfur, phosphorus, etc.), which reduce its quality and deteriorate properties.
The Bessemer and Thomas methods of steel smelting are not used. They have been completely replaced by the converter method - the oxygen-converter method, in which steel is produced in converters with a main lining by blowing the cast iron from above with technically pure oxygen.
The technological cycle for smelting oxygen-converter steel is 50–60 minutes, and the duration of oxygen purging is 18–30 minutes.
In modern converters, the finished metal is released not through the neck, but through a tap hole, which eliminates contact of the metal with air and protects it from the absorption of nitrogen and other gases, since the entire surface of the steel in the converter is covered with a layer of slag during the tapping period.
The disadvantages of the oxygen-converter method of steel smelting include: large dust formation caused by abundant oxidation and evaporation of iron; metal waste (6–9%) is significantly greater than with other methods of steel smelting. In this regard, expensive and complex dust cleaning installations are necessarily installed at converters.
MARTEN METHOD
One of the most important advantages of the open-hearth method is the ability to use different batches and a variety of fuels. In addition, this method makes it possible to smelt a wide range of carbon and alloy steels, with the exception of high-alloy steels and alloys. The disadvantages of the open-hearth method are the long duration of the process (several hours) and significant fuel consumption.
The most widespread is the basic open-hearth process. It allows sulfur and phosphorus to be removed from steel during the melting process by introducing lime.
The acid open-hearth method of steel smelting, in contrast to the main open-hearth method, is carried out in furnaces with an acid lining made of silica brick. The flux and slag in these furnaces are acidic. Phosphorus and sulfur are not removed during the acid smelting process, since the flux does not contain free lime. The charge must be pure in terms of sulfur and phosphorus. With this method, steel is deoxidized better and with less consumption of deoxidizing agents. Therefore, acidic open-hearth steel contains less dissolved gases and non-metallic inclusions than basic steel and has higher mechanical properties.
Alloyed high-quality steels are smelted in acid open-hearth furnaces, since the waste (oxidation) of alloying elements in them is less than in basic ones. But acidic open-hearth steel is approximately 1.5–2 times more expensive than basic open-hearth steel.”
The open-hearth method of steel smelting is constantly being improved. Automation of the furnace thermal regime has been introduced to save fuel and also facilitate the work of steelworkers. Two directions have been developed for the use of oxygen in the open-hearth steelmaking process to accelerate it: enrichment of air blast with oxygen to 25–35%, short-term introduction of oxygen into the furnace through water-cooled molds to intensify the oxidation of impurities.
In our country and some foreign countries, double-bath open-hearth furnaces are being introduced. Their performance is close to that of powerful oxygen converters.
NEW METHODS OF STEEL MELTING
All modern steel-making converter units, open-hearth, electric arc and other furnaces are batch units. In Russia and abroad, the scope of work in the field of creating rational designs for continuous steel-smelting units (SAND) is growing to increase the productivity of units, reduce operating costs, improve the quality and uniformity of products, reduce technological waste, and better use of additional materials.
SAND projects include multi- and single-stage processes for smelting steel from cast iron. In multi-stage SAND processes, metal is mixed from one container to another or gradually flows from one part of the unit to another. In this case, one or more technological operations occur in each container or part of the unit, for example, dephosphorization, desulfurization and deoxidation. In single-stage processes, all operations of removing impurities and converting cast iron into steel occur simultaneously or almost simultaneously.
Remelting processes are various methods of remelting conventional ingots (in a converter, electric arc furnace, etc.). The purpose of remelting is to improve the quality of the metal. In industry, remelting processes are sometimes called special metallurgy. Remelting processes include steel remelting in a vacuum induction furnace (VIF), as well as vacuum arc remelting (VAF), electroslag remelting (ESR), electron beam remelting (EBF) and plasma arc remelting (PAF). The most widespread are VDP and ESR.
The VAR process involves remelting an ingot under the influence of high temperatures arising in the zone of an electric arc between the electrode, the ingot being remelted and the mold tray, which is cooled by circulating water. The metal at the end of the electrode (ingot) melts and its drops continuously fall into the crystallizer, forming a new ingot. Before remelting, the installation is vacuumized. A vacuum is created during the entire remelting period. In modern VARs, ingots weighing from several hundred kilograms to 40–50 tons are produced.
The ESR process was developed at the Institute of Electric Welding named after. E. O. Paton and is distributed in many countries. A layer of slag is melted in a water-cooled crystallizer. The pole of a high-power alternating current source is brought to the bottom of the crystallizer. A steel ingot (electrode) is dipped into the slag and connected to the other pole of the current source. The electric furnace is closed between the ingot being melted (electrode) and the crystallizer bath through a layer of molten slag (there is no electric arc). The slag has high electrical resistance and is heated to a temperature of 1700–2000°C. As a result of this, the end of the electrode (ingot) immersed in it melts and molten drops of metal pass through the slag layer, cleared of sulfur. The content of non-metallic inclusions and gases in them decreases. Once on the cold walls of the crystallizer, drops of metal solidify and gradually form a new dense high-quality ingot.
The advantage of the ESR process is its comparative simplicity and low cost and a guaranteed increase in the quality of the metal after its remelting as a result of reducing the sulfur content, non-metallic inclusions and obtaining a dense ingot. In our country, ESR installations produce ingots weighing up to 360 tons.
METHODS OF STEEL CASTING
After smelting and releasing liquid steel into a ladle, it is poured into ingots of various shapes and weights, which serve as blanks for producing products in rolling and forging shops. Steel is poured in two ways: into special molds - molds for producing ingots and by continuous casting in continuous steel casting plants.
Pouring ladles consist of a durable casing with trunnions for gripping by a crane. There is a hole in the bottom of the ladle through which liquid steel is poured during casting. This hole is closed from the inside of the bucket with a stopper through a system of levers located on the wall of the bucket. The inside of the bucket and stopper are lined with fireclay bricks.
The steel tapped from the furnace into the casting ladle is kept in the ladle for 5–10 minutes to level out its composition and the floating of non-metallic inclusions and gases present in it.
Molds of two shapes are used: with widening at the top and the presence of a bottom for casting mild steel; with widening downward without a bottom (through) for casting boiling and semi-quiet steel. Molds without a bottom are placed on pallets when pouring.
Ingots for long products (angles, I-beams, etc.) have a round or square cross-section, ingots for rolling into sheets are flat.
Molds are filled in various ways: from above, directly from the ladle or from below, through a vertical sprue using a siphon method. From a vertical sprue in the siphon method, molten steel flows through horizontal gating passages from below into several molds, filling them from the bottom up according to the principle of communicating vessels. Due to the simplicity and absence of metal loss in the form of sprues, pouring steel from above into molds is often preferred. Since casting from above is more economical than siphon casting, it is used for carbon and other grades of steel. But the surface of these castings is uneven, with films, so after rolling it requires additional cleaning.
High-quality and alloy steels are cast mainly using the siphon method to avoid the loss of expensive metal due to surface cleaning. Siphon casting usually produces small ingots weighing up to 2.5 tons. Most often, ingots are cast weighing 1–20 tons, and in some cases – 100 tons or more.
When casting calm steel, profitable extensions are installed on top of the molds, lined from the inside with a refractory mass, which leads to a longer preservation of the liquid state of the steel in them, allowing the ingot to be fed with metal during shrinkage. As is known, during hardening the metal decreases in volume and shrinks. Due to insulation, the profitable part of the ingot freezes last and all non-metallic contaminants and shrinkage voids are concentrated in it, and the entire ingot remains practically clean.
Continuous casting of steel to produce ingots is carried out in continuous casting installations of vertical and radial types. The vertical type installation is a multi-story structure. From the casting ladle, through an intermediate tundish, also in the form of a ladle, steel is poured into the mold in a continuous and uniform stream. It is made in the form of a copper box with a double wall. The crystallizer swings slightly up and down and is intensively cooled by running water, which leads to the formation of strong and dense steel ingots. From the crystallizer, the ingot is gradually and continuously pulled out by rollers at a speed equal to the rate of crystallization of the ingot. At the exit from the crystallizer, in front of the pulling rollers, the continuous ingot enters a secondary cooling zone, where it is cooled by water showers, resulting in its complete solidification. At the exit from the rolls, the continuous ingot is cut into individual ingots of the required length by a gas cutter.
A radial type continuous casting plant is more suitable for placement in modern steelmaking shops. The principle of its operation is similar to that discussed.
Continuous steel casting plants facilitate the working conditions of workers and increase the productivity of steel casting and further processing of steel ingots. Depending on the cross-section and shape of the ingots, a single-strand continuous casting plant can produce 20–150 t/h of ingots.
ELEMENTS OF METAL SCIENCE
Metals and their alloys in the solid state are crystalline bodies in which atoms (positively charged ions) are arranged in a certain regular order. The forces of their mutual attraction and repulsion are balanced and the solid body retains its shape. The correct, regular arrangement of atoms in space determines the crystalline structure of metals and alloys.
The crystal lattices of different metals and alloys are different. The simplest cell of a crystal lattice is cubic. Atoms (ions) are located at the vertices of the cube, touch each other and oscillate near equilibrium points, i.e. near lattice nodes with high frequency. Most often, in metals and alloys, as well as in iron and steel, there are more densely packed cells of crystal lattices: body-centered cube (BCC), face-centered cube (FCC), hexagonal close-packed (HCP), etc. BCC crystalline cell (Fig. 1, a) has atoms at all vertices of the cube, as well as one atom at its center at the intersection of the diagonals of the cube. The fcc crystal cell (Fig. 1, b) is characterized by the arrangement of atoms at the vertices of the cube and at the center of each of its faces at the intersection of its diagonals. In a cell of a hexagonal lattice, atoms are located at the vertices of the hexagonal bases of the prism, at the centers of these bases and inside the prism (Fig. 1, c).
Under real conditions, metal crystals exhibit one or another deviation from the correct crystallographic structure of their lattices. These imperfections are usually called defects in the crystal structure of metals and alloys. These include point defects (vacancies or free sites in the crystal lattice), linear defects (dislocations), etc.
Rice. 1. Unit cells of crystal lattices: a – body-centered cube (BCC);
b
– face-centered cube (FCC);
c
– hexagonal close-packed (hcp);
a, c, d
– lattice parameters
Rice. 2. Iron cooling curve
Some metals, such as iron, cobalt and others, and, consequently, alloys based on them, when heated and cooled to different temperatures in the solid state, can have a different crystal structure with a specific crystal lattice in a given temperature range. This phenomenon of the existence of one metal in several crystalline forms is called allotropy, or polymorphism. The various crystalline forms of a metal are called allotropic, or polymorphic, modifications.
Rice. 3. Cooling curves for pure metals:
1 - theoretical;
2
and
3
- experimental
Allotropic modifications are denoted by Greek letters (α, β, γ, δ, etc.) added to the symbol corresponding to the given element.
Solid iron at different temperatures exists in two allotropic modifications: Feα and Feγ. In accordance with this, the cooling curve of iron (Fig. 2) has a different form compared to the cooling curve of pure metal without allotropic transformation in the solid state (Fig. 3).
The transformation of one allotropic form into another is accompanied by the release of latent heat of crystallization upon cooling. Therefore, horizontal plateaus are observed on the cooling curve of iron at temperatures of allotropic transformation. The upper branch of the cooling curve (see Fig. 2) characterizes the cooling of liquid iron. At a temperature of T = 1539 °C, a horizontal plateau is observed, which corresponds to the crystallization of the allotropic modification of Feα. It has a bcc crystal lattice, its own structure and properties, and is non-magnetic. The branch of the cooling curve in the temperature range 1539-1392 °C is the cooling of the solid modification Feα(δ) · At a temperature of 1392 °C, a second horizontal plateau is observed, which corresponds to recrystallization in the solid state of one allotropic modification into another: Feα → Feγ. The Feγ modification has an fcc crystal lattice (face-centered cube), its own structure and properties, and is non-magnetic.
Next, Feγ cools, which is characterized by a branch of the cooling curve in the temperature range 1392-911 °C. At a temperature of 911 °C, a third horizontal plateau is observed on the cooling curve, which corresponds to a new recrystallization of iron in the solid state with rearrangement of the crystal lattice. Here the transition of Feγ to Feα occurs. Iron Feα has a bcc crystal lattice, its own new structure and properties, and starting at a temperature of 768 ° C (Curie point) it becomes magnetic. The lower branch of the cooling curve from 911 °C to room temperature is the cooling of a new solid modification of iron – Feα. At a temperature of 768 °C, an inflection in the cooling curve is observed, which corresponds to the appearance of magnetic properties in iron. There is no change in structure, mechanical and physical properties at this point.
Cooling of a liquid metal that does not have polymorphism is accompanied by a smooth decrease in temperature (upper branch of the cooling curve) (see Fig. 3). During this period, no qualitative change in the state of the metal is observed, therefore it is called simple cooling of the metal. When the theoretical crystallization temperature Ts is reached, horizontal areas appear on the cooling curves, indicating that the metal temperature during the crystallization period remains constant. This is explained by the release of latent heat of crystallization during crystallization, which compensates for the heat removal during the cooling process of the metal. The length of the horizontal section of the cooling curve corresponds to the time of crystallization, indicating the beginning and end of the process. Upon completion of crystallization, i.e. when the transition of the metal from the liquid to the solid state is completed, the temperature decreases again - the metal cools in the solid state (the lower branch of the cooling curve). In real conditions, the solidification process can only occur when the metal is supercooled to the actual crystallization temperature Tn (where n is the degree of supercooling), which lies below the theoretical crystallization temperature Ts. The difference between the theoretical and actual crystallization temperatures of the metal is called the degree of supercooling n. On curve 3 there is a jump in the increase in Tn due to the rapid release of the latent heat of crystallization at its first moment.
In industry, mainly iron-based alloys, called steels and cast irons, are used for building structures and gas and oil pipelines. To study the state of alloys of different concentrations at different temperatures, phase diagrams are used - a graphical representation of the phase state of alloys depending on temperature and concentration under equilibrium conditions at constant pressure. They are constructed experimentally using cooling curves for alloys of different concentrations.
The substances that make up an alloy are called components.
The structure of alloys is more complex compared to pure metals. The properties of alloys are very diverse and depend on what components make up the alloy, in what quantities and what interactions they enter into in liquid and solid states. Therefore, alloys are most widely used in technology compared to pure metals.
The components in the alloy may not interact with each other and form in the solid state a mechanical mixture of crystals of both components, crystallizing simultaneously (Fig. 4, a); dissolve in each other and form liquid and solid solutions (Fig. 4, b); enter into a chemical interaction and form a chemical compound, as well as intermediate and complex phases (for example, a solid solution based on a chemical compound). In microstructure, solid solutions, like pure metals, are homogeneous grains.
Rice. 4. Schemes of microstructures of alloys: a - mechanical mixture of components
A
and
B;
b – solid solution of components
B
and
A
The solid solution is single-phase and consists of one type of crystal lattice. It is formed on the basis of one of the components called the solvent metal. The crystal lattice of the solvent metal contains atoms of another component called the solute.
Solid solutions are divided into substitutional and interstitial solutions depending on the nature of the arrangement of atoms of the solute in the crystal lattice of the solvent (Fig. 5). In solid substitution solutions, the atoms of the dissolved component B partially replace the atoms of the crystal lattice of the solvent component A. In interstitial solid solutions, the atoms of the dissolved component C are introduced into the crystal lattice of the solvent component A, located between the atoms A. Substitution solid solutions are limited and unlimited. With unlimited solubility, atoms of solvent A can be replaced by atoms of solute B. This is possible if both components have the same structure and crystal lattices, a small difference in the atomic sizes of the components, and also valence shells of atoms that are similar in structure and physical nature.
Rice. 5. Solid solutions based on metal with a bcc crystal lattice:
a – pure metal;
b
– substitutional solid solution;
c
– interstitial solid solution
A solid solution based on a chemical compound is a substance in the crystal lattice of which there may be atoms of another element that has replaced the atoms of one of the components. For example, instead of Fe3С there will be (Fe, Μn)3С.
A phase is a part of a system that is homogeneous in chemical composition, crystalline structure, and properties, separated from other parts of the system by an interface. The phases can be a liquid solution of alloy components, their solid solution, a chemical compound, or crystals of pure components. A system is a set of phases that are in equilibrium under certain external conditions (temperature, pressure). A single-phase system is, for example, a homogeneous liquid solution of components (liquid). An example of a two-phase system is a mechanical mixture of crystals of two components. Their stable chemical compounds can also be used as components. The internal structure of metals and alloys is characterized by micro- and macrostructure. Microstructure is the internal structure of metals and alloys, studied under a microscope at high magnifications (50-2000 times). It is determined by the shape and size of the phases, their relative position. Macrostructure is the internal structure of metals and alloys, studied at low magnifications using a magnifying glass (up to 10-30 times) or with the naked eye (visually). It is used to determine various metal defects, heterogeneity of its structure, etc.
The type of phase diagram depends on the interaction of components in the liquid and solid states. If the system is one-component, for example pure metal, then the phase diagram will have one ordinate axis - the temperature scale. One point will be marked on it, corresponding to the equilibrium temperature of transition from a liquid to a solid state of aggregation (and vice versa, from solid to liquid) of pure metal Tn. This equilibrium melting point is determined by its cooling curve. If there are two components in the system, then the second axis, the abscissa axis, will be the alloy concentration scale, since the phase diagram of a two-component alloy is constructed in two dimensions (temperature - concentration).
Rice. 6. State diagrams of a one-component system (a) and a two-component system with unlimited solubility B and A in solid and liquid states (b) :
L – liquid solution; α – solid solution of B in A; L + α – two-phase region; e – alloy of a given concentration of 25% B at a given temperature T1
This state diagram of alloys has two vertical temperature scales, corresponding in concentration to the first A and second B components (Fig. 6). Each point on the phase diagram characterizes the state of the alloy of a given concentration at a given temperature; each vertical corresponds to an alloy of a given concentration, considered at different temperatures.
Rice. 12. Scheme of formation of structures during graphitization
On the liquidus line to the left of point C'
When alloys are cooled, austenite is released from the liquid, and graphite is released to the right of point
C'
.
The process of graphite formation is called graphitization. For graphitization processes to occur in accordance with the iron-graphite phase diagram, cooling must be slow. With faster cooling, white cast irons are formed. In addition to the considered process of graphite formation, during the crystallization of iron-carbon alloys, another method of graphite formation can be observed as a result of the decomposition of cementite when heated. Cementite Fe3C is an unstable compound. Under certain conditions (temperature), it decomposes into ferrite and graphite: C → F +
G.
Let's assume that the cooling was fast enough and the result was white cast iron consisting of ledeburite (perlite + cementite). For graphitization, white cast iron is heated above the P'S'K'
(Fig. 12), as a result of which the transformation
P + C → A + C
.
During exposure at high temperatures, cementite decomposes with the release of graphite: C → A + G
(1st stage of graphitization).
When cooling below the P'S'K'
A → P
occurs , and if complete cooling is carried out, the structure of cast iron will consist of
Π
+
G
(straight line
3)
.
Such cast iron is considered to have a pearlite base. If cast iron is kept at a temperature below the P'S'K'
, then the cementite in pearlite will disintegrate
(CP→ F + G)
partially or completely with longer exposure (And the graphitization stage).
As a result, the structure of cast iron can consist of P + F + G
or
Φ + G
when completely cooled (straight lines
4
and
5
).
These cast irons are called pearlite-ferritic and ferritic cast irons, respectively. If white cast iron is heated above the Ρ'S'K'
and immediately cooled, it will acquire the original structure
P + C
(straight line 7).
If white cast iron is heated above the Ρ'S'K'
and held for a short time at this temperature, then partial decomposition
C → A + G
, and after complete cooling the cast iron will have the structure
C + Π + G
(half cast iron) (straight line
2
) .
Rice. 13. Microstructures of gray cast iron with plate-shaped graphite on a ferritic (a), ferrite-pearlitic (b), pearlitic (c) basis and with vermicular graphite (worm-shaped) on a ferritic (d), ferritic-pearlitic (e), pearlitic (e) basis ) basis; malleable cast iron with flake graphite on a ferritic (g), ferritic-pearlitic (h) and pearlitic (i) basis; high-strength cast iron with nodular graphite on a ferritic (k), ferritic-pearlitic (l) and pearlitic (m) basis
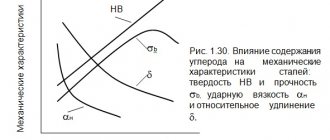
Cast irons with graphite inclusions are mainly divided into gray, malleable and high-strength cast irons. as well as their other varieties, in which free carbon in the form of graphite is located on a ferrite, pearlite or ferrite-pearlite base (Fig. 13). In gray cast irons, graphite is present in the form of veins (flakes), in malleable cast irons - in the form of flakes, and in high-strength cast irons - in a spherical (spherical) form. Due to changes in the shape of graphite inclusions in cast iron, their properties change significantly. Cast iron, like steel, is an alloy of iron and carbon (2.14–6.67%) in combination with various impurities (manganese, silicon, sulfur, phosphorus, etc.), but with a higher content.
Gray cast iron is marked with the letters СЧ and two-digit numbers. For example, SCh15, where the number shows the value of the tensile strength: σв = 150 MPa. The larger the graphite veins in gray cast iron, the worse its mechanical properties.
To obtain malleable cast iron, white cast iron (hypoeutectic) is subjected to prolonged heating (annealing), which leads to the formation of graphite inclusions in it in the form of flakes (see Fig. 13, d–f
). Malleable cast irons have better mechanical properties than gray ones, as they have some ductility. They are marked with the letters KCH, and then with numbers, which also indicate tensile strength.
High-strength cast irons are produced by introducing small additives, such as magnesium, into a ladle with liquid cast iron, which leads to the formation of spherical graphite inclusions (see Fig. 13, g
). It is marked with the letters HF and numbers indicating tensile strength. For example, HF40, HF45, HF100. High-strength cast iron with nodular graphite has properties similar to steel; it has a certain margin of ductility and even viscosity, depending on the structure (grade).
The production of carbon in cast iron in the form of graphite of various shapes and sizes, as well as the introduction of alloying elements into them, leads to a change in their mechanical properties and in some cases allows them to replace steel castings and forgings with almost equivalent mechanical properties.
Previous1Next
WHAT HAPPENS WHEN WE FIGHT Without understanding the differences that exist between men and women, it is very easy to lead to a quarrel...
Conflicts in family life. How can I change this? It is rare that a marriage and relationship exists without conflict and tension. Everyone goes through this...
WHAT HAPPENS IN ADULT LIFE? If you are still connected to your mother in the wrong way, you are avoiding separation and independent adult existence...
What will happen to the Earth if its axis shifts by 6666 km? What will happen to the Earth? - I asked myself...
Didn't find what you were looking for? Use Google search on the site:
Characteristics of types of carbon metal
The iron-carbon diagram shows what cast iron is made of. In addition to iron, carbon is present in the form of graphite and cementite.
The composition of the cast iron alloy has varieties:
- White. The carbon present here is in a chemically bound state. The metal is strong, but brittle and therefore difficult to machine. In industry it is used in the form of castings. The properties of the material allow it to be processed with an abrasive wheel. The welding process causes difficulty, since there is a possibility of cracks due to the heterogeneity of the structure. Found application in areas related to dry friction. Has increased heat resistance and wear resistance.
- Half-hearted. It has increased fragility, so it is not widely used.
- Grey. GOST 1412–85 indicates what percentage of impurities this metal contains: 3.5% carbon, 0.8% manganese, 0.3% phosphorus, 0.12% sulfur and up to 2.5% silicon. The carbon present in the platelet form creates low impact strength. The characteristics of the type indicate that the material works better in compression than in tension. When heated sufficiently, it has good weldability.
- Malleable. A ferrite base of this type provides it with high ductility. When broken, it has a black, velvety color. It is obtained from white, which languishes for a long time at a temperature of 800-950 degrees.
- Highly durable. The difference from other types is the presence of spherical graphite. It is obtained from gray after adding magnesium to it.
Individual properties of metal
The material is characterized by certain characteristics. These include:
- Physical. Values such as specific gravity or expansion coefficient depend on the carbon content of the metal. The material is heavy, so cast iron bathtubs can be made from it.
- Thermal. Thermal conductivity allows you to accumulate heat and retain it, distributing it evenly in all directions. This is used in the manufacture of frying pans or radiators for heating.
- Mechanical. These characteristics vary depending on the graphite base. The most durable is gray cast iron with a perlite base. A material with a ferrite component is more malleable.
Depending on the presence of impurities, a difference in the properties of the material appears.
These elements include sulfur, phosphorus, silicon, manganese:
- Sulfur reduces the fluidity of the metal.
- Phosphorus reduces strength, but makes it possible to manufacture products of complex shapes.
- Silicon increases the fluidity of the material, lowering its melting point.
- Manganese gives strength, but reduces fluidity.
Slag formation
Thus, we have found out how cast iron is produced. However, when this material is smelted, another product is obtained that is widely used in the national economy. When melting 1 ton of cast iron, 0.6 tons of slag comes out. The fact is that even refined iron ore contains a fairly large amount of clay. Coke also contains ash. To remove these unnecessary elements, among other things, fluxes (calcium and magnesium carbonates) are added to the charge. During the smelting process, they enter into a chemical reaction with various types of impurities, resulting in the formation of slag. It is an aluminosilicate or silicate melt.
The density of slag is less than that of liquid cast iron. Therefore, during the melting process it is located underneath. It is removed periodically through a separate taphole, called a slag taphole. This by-product of iron foundries is used mainly for the production of cement and building blocks as a filler.
Agglomeration process
In fact, we’ll look at how cast iron is produced below. Now let's talk about how ore is prepared for smelting directly at metallurgical plants.
If ordinary crushed material is used for smelting, the productivity of the blast furnace will drop sharply. The fact is that such a charge has a low degree of gas permeability. Therefore, before loading into the blast furnace, ore must undergo an agglomeration process.
This procedure is carried out in specialized workshops of metallurgical plants and is a process of sintering rock into pieces of a certain size most suitable for smelting cast iron. Adhesion occurs at a high temperature sufficient to easily melt the surface of the charge particles. As a result, the latter simply stick together to form pieces. In this case, the ore is first mixed with coal. As a result of the combustion of the latter, the temperature necessary for obtaining pieces is achieved. The agglomeration process is stimulated by passing air flows through the layer of ore with coal (from top to bottom).
Not only ore can be used to obtain sinter. Sometimes it is also made from small pieces of iron. Its alloy with what substance makes it possible to obtain cast iron will be discussed below. Of course, it is not pig iron that is used to produce this metal. Regular scrap metal is melted down into cast iron.
Mining and processing plants
The main raw material used in the production of cast iron is iron ore. It is mined in quarries in different places in our country. As you know, mined ore contains a large amount of various types of impurities. Of course, it cannot be used for melting cast iron in such a “raw” form. Therefore, at the first stage, it goes to special enterprises - mining and processing plants. Here waste rocks are removed from it and crushed. Then the clean ore is loaded into train cars and sent to metallurgical plants.
What happens in the oven
So, let's look at how pig iron is produced in a blast furnace. The inside of a stove of this design is lined with brick. The principle of its operation is relatively simple. In the production of cast iron, in addition to sinter, coke, lime and flux are used. A mixture of these materials is prepared in a certain proportion. This is what is called a blast furnace charge. It is poured into special lifts and raised to the very top of the furnace.
In order for coke to ignite, a large amount of air enriched with oxygen is necessary. It is fed into the blast furnace from below, through special openings called tuyeres. It is blown into the furnace under very high pressure. This is necessary so that air penetrates through the layer of charge supplied from above. In this case, the flow is preheated to 600-800 degrees, otherwise the temperature inside the furnace will drop.
The cast iron obtained by straightening the charge flows down and is released out through a special hole called a taphole at intervals of approximately once every 40 minutes. Next, it is poured into large-capacity bowls and transported to steelmaking shops.
Restoring other items
Mn, silicon, sulfur and phosphorus enter the blast furnace along with the charge in the form of various chemical compounds. Higher manganese oxides are reduced to MnO according to approximately the same principle as iron: MnO2 - Mn2O3 - Mn3O4 - MnO. Pure manganese is released as follows: MnO + C = Mn + CO - Q. Silicon enters the furnace in the form of silica SiO2. Its reduction occurs by the reaction SiO2 + 2C = Si + 2CO - Q.
Phosphorus is reduced by hydrogen, solid carbon and CO and, unfortunately, goes into cast iron almost completely. This element deteriorates the blast furnace iron alloy. Allows you to obtain good quality cast iron with silica present in the charge, as well as higher manganese oxides. In some cases, Mn is added to the blast furnace on purpose. This produces a special type of cast iron - manganese.