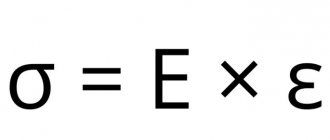Rubber: pixabay.com The milky sap of some plants contains latex. It is on the basis of this white liquid that rubber is created. This material is elastic, it is not permeable to water and does not conduct electricity. Today, not only natural rubber is produced, but also its various synthetic analogues. All of them are raw materials for the production of insulating materials, shoes and clothing, and tires.
History of discovery
This substance has been known to mankind for many hundreds of years. It is known that the Incas and Mayans made balls from rubber for playing ball. Archaeologists found them during excavations, and their age reached 900 years.
Europeans learned about this material much later. Columbus in 1493 in Haiti saw the natives playing with a ball made of rubber.
When the Spaniards took them in their hands, they found that the rubber was sticky and heavy, and smelled of smoke. To make such balls, local residents collected milky juice from the Hevea tree. Balls were rolled out of it and the product was allowed to thicken.
The use of unusual material was not limited to this. The Indians made galoshes from it. Although they did not allow water to pass through, in the heat they began to melt and stick to their feet. If it turned out that these shoes stretched, then they never compressed enough to fit their previous size.
Columbus brought samples of rubber to Europe, but for a long time there it was not possible to produce objects similar to those used by the Indians.
For two centuries, this material remained a curiosity until, in 1730, British chemist Joseph Priestley discovered that rubber could erase what was written with a graphite pencil. In 1791, a businessman from England, Samuel Peale, received a patent for a method he invented for treating clothing, making it waterproof using rubber. Beginning in 1820, France learned to use this material to make garters for women and suspenders for men. For this purpose, rubber threads were used, which were woven with fabric.
British scientist Charles Mackintosh came up with the idea that a layer of rubber could be placed between layers of fabric and thus create a waterproof material for making raincoats. In 1823 he began the production of such clothing. Unfortunately, a cloak made in this way could not withstand cold or heat. In the first case, it became stiff, and in the second, it began to creep apart.
Scientists began to look for ways to make a material from rubber that would be devoid of these disadvantages. American inventor Charles Goodyear solved this problem in 1839 by adding sulfur to rubber.
It turned out that if you put a fabric covered with rubber on the stove, and then apply a layer of sulfur and heat it up, the resulting material will be free from these disadvantages.
The enrichment of rubber with sulfur became known as vulcanization. As a result, rubber was obtained, which began to be actively used. By 1919, there were about 40 thousand different types of rubber products.
The difference between rubber and rubber is as follows:
- rubber has a high level of elasticity, strength, and resistance to adverse effects;
- rubber is valuable primarily not for its performance qualities, but for the fact that it is a raw material for the production of rubber.
Do you know which city produces rubber in Russia? This is Yaroslavl. The plant has been operating since 1932.
Physico-chemical properties of rubber
This material is an elastic mass that was originally obtained from the Hevea plant. Over time, the milky sap coagulates and forms a viscous material. To prevent this from happening, sodium hydrosulfide or formaldehyde is added to it.
Freshly extracted rubber juice (latex) is characterized by the following properties:
- The specific gravity is 0.9794 (with a rubber content of 35 g per 100 cubic cm).
- At a temperature of 30 degrees Celsius, the viscosity ranges from 12 to 15.
- The size of rubber particles is 0.5-5 microns. In 1 cubic cm of juice, their number reaches 200 million.
Rubber is a polymer of unsaturated hydrocarbons. Its chemical formula is as follows: (C5H8)n - it is an isoprene polymer. The molecular weight of this substance is 15000-30000. After conducting research, scientists found that rubber consists of a polymer of 2-methylbutadiene.
Natural rubber
99% of this material is obtained from the Hevea tree. To do this, cuts are made on the bark in the shape of the letter V. A groove is installed in the lower part, perpendicular to the surface, through which the juice gradually flows into a bowl placed below. The leakage of latex (milky sap of Hevea) lasts for one and a half hours.
The rubber content in it may vary. It depends on the:
- the age of the tree from which the sap is collected;
- the composition of the soil in which Hevea grows is important;
- the time of year when the collection takes place;
- what the weather was like at that time;
- time and quality of cuts made;
- other features of latex collection.
In order for natural rubber to be used, it must undergo the following processing:
- First the spin is done. It is necessary in order to remove excess moisture from latex.
- After this, the resulting strips are wrapped around a stick and dried over a fire.
- The strips are laid out in one layer and left in the sun.
- Now all that remains is to hold it over the smoke.
Rubber prepared in this way can serve as a raw material for rubber production.
The juice is extracted from trees that are already 12 years old. From 3 to 5.5 kg of latex can be obtained per year.
Composition of latex solution:
- up to 70% water;
- the rubber content in different cases ranges from 25% to 70%;
- the content of other chemicals, including protein, does not exceed 1-2%.
Rubbers are natural or synthetic products of the polymerization of certain diene hydrocarbons with conjugated bonds. The most important physical properties of rubbers are elasticity (the ability to restore shape) and impermeability to water and gases.
Rubbers are elastic high-molecular materials (elastomers), from which rubber is produced by vulcanization (heating with sulfur).
Polymers obtained from unsaturated hydrocarbons, including artificial rubbers, are especially important. All rubbers are divided into natural and synthetic, the latter, in turn, depending on the substance used for synthesis, are divided into butadiene, isoprene and chloroprene rubbers.
Natural rubber or gutta percha
Natural rubber is obtained from latex - the milky juice of the Hevea plant. To make it flow out, V-shaped cuts are made on the tree bark. From a healthy tree, latex can be harvested for 30 years. The Indians called it “kaw choo”, i.e. "tears of a tree"
Harvesting rubber from the Hevea plant
History of the discovery and use of rubber
Natural (natural) rubber in chemical composition is a high-molecular unsaturated hydrocarbon of the composition (C5H8)n, where n is 1000-3000 units. When heated without access to air, rubber decomposes to form a diene hydrocarbon - 2-methylbutadiene-1,3 or isoprene.
Rubber in which all the elementary units are in either a cis or trans configuration is called stereoregular.
Video “Natural rubber”
Natural rubber is a stereoregular polymer in which isoprene molecules are connected to each other according to a 1,4-addition scheme with the cis configuration of the polymer chain:
cis -polyisoprene (rubber)
Under natural conditions, natural rubber is not formed by polymerization of isoprene, but by another, more complex method.
The molecular weight of natural rubber ranges from 7·104 to 2.5·106.
The trans-polymer of isoprene also occurs in nature as gutta-percha:
The cis form is more elastic, because easily curls into a ball.
The trans form is less elastic, because macromolecules are more elongated.
The most important physical property of rubber is elasticity, that is, the ability to reversibly stretch under the influence of even a small force. Another important property is impermeability to water and gases. The main disadvantage of rubber is its sensitivity to high and low temperatures. When heated, rubber softens and loses elasticity, and when cooled, it becomes brittle and also loses elasticity.
These disadvantages can be overcome by heating the rubber together with sulfur. This process is called rubber vulcanization.
Synthetic rubbers
The first synthetic rubber obtained using the method of S.V. Lebedev, during the polymerization of divinyl under the influence of metallic sodium, was a polymer of irregular structure with a mixed type of 1,2- and 1,4-addition units:
In the fifties, domestic scientists carried out the catalytic stereopolymerization of diene hydrocarbons and obtained stereoregular rubber (structural units and functional groups are located in space in a certain order), similar in properties to natural rubber.
Currently, the industry produces rubber in which the content of isoprene units connected at position 1,4 reaches 99%, whereas in natural rubber they are 98%.
In addition, the industry produces synthetic rubbers based on other monomers - for example, isobutylene, chloroprene, and natural rubber has lost its monopoly position.
Educational film "Rubber"
Educational film "Rubber"
Vulcanization of rubbers
To improve the quality of natural and synthetic rubbers, they are turned into rubber.
Rubber is vulcanized rubber with a filler (carbon black). The essence of the vulcanization process is that sulfur atoms attach to linear (thread-like) rubber molecules at the site of double bonds and, as it were, cross-link these molecules to each other with disulfide bridges, forming a three-dimensional network polymer:
As a result of vulcanization, sticky and weak rubber turns into elastic and elastic
. Rubber is stronger than rubber and more resistant to temperature changes.
Rubbers in the form of rubbers filled with active carbon black are used for the manufacture of car tires and other rubber products.
Depending on the amount of crosslinking agent (sulfur), meshes with different crosslinking frequencies can be obtained.
To vulcanize rubber, a little sulfur is taken, 2 - 3% of the total mass. If you add more than 30% sulfur to rubber, it will join along the line of break of almost all π-bonds and an extremely cross-linked natural rubber will be formed -
, which is not elastic and is a hard material.
The most widespread use of rubbers is the production of rubber for automobile, aircraft and bicycle tires.
Special rubbers of a huge variety of seals are made from rubbers for the purposes of heat-sound-air-waterproofing of detachable elements of buildings, in sanitary and ventilation technology, in hydraulic, pneumatic and vacuum technology.
Rubbers are used for electrical insulation, the production of medical devices and contraceptives.
In rocket technology, synthetic rubbers are used as a polymer base in the manufacture of solid rocket fuel, in which they play the role of fuel, and as a filler, nitrate powder (potassium or ammonium nitrate) or ammonium perchlorate is used, which plays the role of an oxidizing agent in the fuel.
Alcadienes
Synthetic rubber and its main types
Butadiene rubber is used to make automobile tubes and tires. The operational, as well as physical and chemical properties of the products are much better compared to natural materials.
One of its features is the ability to reliably hold air. It exceeds the similar quality of natural material by about 10 times. Chemistry has made it possible to create materials whose characteristics are significantly superior to natural rubber.
Another area of application is the production of ebonite or chemically resistant rubber.
Chloroprene rubber is supplied to customers in the form of a light yellow mass.
Distinctive qualities of the product:
- high resistance to fire and temperature;
- it is resistant to ozone, low temperatures and other types of weather influences;
- it has a high level of adhesion to fabrics, metals and other materials.
The material can crystallize under tension. This quality increases its strength characteristics.
Material made on the basis of ethylene propylene is used where impact-resistant rubber is needed.
Organosilicon rubbers have increased resistance to temperature, chemical influences, and abrasion. This material does not allow gases to pass through.
Divinyl rubber is used to create gaskets in high pressure installations.
Rubber: properties
What qualities does natural rubber have? This polymer is absolutely unique and changes its properties depending on the ambient temperature. It can be highly elastic, fluid, and even glassy.
Rubber: pixabay.com
In the range from 20 to 30 °C the material is characterized by:
- white color or lack of color;
- amorphous loose structure;
- ability to dissolve only in gasoline, benzene, ether;
- insolubility in water and alcohols.
Among the important properties of rubber it should be noted:
- firmness and elasticity. A rubber product can be stretched by 1000%, and even after that it quickly returns to its original state. This quality is lost only during very long storage;
- soft at room temperature and plastic when heated. If you choose the right conditions for working with the material, then the shape obtained during its heat treatment can be preserved;
- impermeable to electricity, heat, gases and water. This property makes the use of rubber very convenient in all areas. Products made from it have a long shelf life and are little susceptible to environmental influences.
Rubber: pixabay.com
This is why none of the previously known materials could compare with rubber, much less compete with it.
Obtaining synthetic rubber
When rubber began to be widely used in industry, there was an acute shortage of natural rubber for its production. This situation has confronted scientists with the task of synthesizing an artificial material with the same physical and chemical properties.
Preparation of synthetic rubber using the Lebedev method
The installation for producing this material was first put into operation in the thirties of the 20th century.
Synthetic rubber is made from divinyl, which is extracted using the decomposition reaction of alcohol. The monomer of artificial rubber is isoprene. The material is obtained by polymerization.
Test on the topic
- /5
Question 1 of 5When was artificial rubber first produced?
Start test
Hall of Fame
To get here, take the test.
- Irina Volkova
5/5
- Tarek El-Gohary
5/5
- Mikhail Ilyushin
5/5
- Yana Akbash
4/5
Rubber Applications
In its pure form, this material is rarely used. In most cases, it is used as a base for making rubber.
After rubber was brought to Europe, until the 18th century, rubber was considered simply one of the overseas curiosities. Elasticity and water-repellent properties made it possible to use the material for the manufacture of shoes and clothing that do not allow water to pass through, however, low performance qualities prevented its spread.
After the discovery of vulcanization of rubber, which made it possible to make rubber, the use of the new material became very common. Gradually, the quality of rubber improved and a large number of different products began to be made from it.
Examples include:
- tires;
- children's rubber toys;
- shoes;
- clothes;
- electrical insulation for wires;
- conveyor belts;
- medical products;
- rubber protective gloves.
Now it is difficult to name an area of human life where rubber is not used.
Natural rubber continues to be used today. Tires, shock absorbers, and some products for sanitary and hygienic purposes are made from it.
Industrial Application
The most widespread use of natural rubber in practice is the production of rubber. This process is based on the vulcanization reaction, developed back in the 19th century.
To produce rubber, various components are added to the raw material to promote the formation of long molecules interconnected by cross-links. This structure provides rubber with the ability to compress and stretch at almost any temperature.
Industrial applications of natural rubber
The product of vulcanization - rubber is intended for use in various industries. E is used for the production of tires and tubes for any equipment operating on wheels.
In addition, rubber serves as the basis for the production of various seals used for heat, water and sound insulation. Medicine cannot do without it, in particular in the production of gloves and condoms. In addition, many products made from it are used in medical devices and equipment.
Tires made of natural rubber Natural rubber as a sealant
Rubber is also used in industries such as rocketry. It is used as a basis for the production of solid fuel for rockets. In particular, it is used as fuel, and the filler is saltpeter powder, and the oxidizing agent is ammonium perchlorate.
Interesting facts about rubber
After the vulcanization process was discovered, the material began to be actively used in industry. At the same time, the Hevea juice, which was mined in the Brazilian jungle, became scarce.
In order to increase rubber production, large Hevea plantations were established on the islands of Java and Sumatra.
Although the main source of natural rubber is Hevea, nevertheless, in nature there are other options for obtaining this raw material from plants.
Types and types of natural rubber:
Natural rubber is divided into 8 types, forming 35 varieties.
The most common and valuable type of natural rubber is “ smoked sheet ,” which means smoked leaf. It is made in the form of fairly transparent amber-colored sheets with a corrugated surface.
Less common is the type called " light crepe ". To obtain it, sodium bisulfite is added to the latex before gelatinization for bleaching. Sheets of this type of rubber are cream-colored and opaque.
The least valued type is called " para-rubber ". It is extracted from wild Hevea using an artisanal method.