Profit Steel
Stainless steel is a popular material in the food industry, mechanical engineering, construction and other industries. Such steel...
Stainless steel is a popular material in the food industry, mechanical engineering, construction and other industries. This steel is corrosion-resistant, durable, and parts made from it do not require additional processing. Before purchasing it, it is important to check whether this metal is a stainless steel - this can be done in several ways without involving specialists.
Stainless steel is distinguished by properties, type, marking and other parameters. The assessment will require a sample of steel that can be tested. Before checking, you need to study the main features of such metals. Let's talk about this in more detail.
PROPERTIES AND CHARACTERISTICS OF STAINLESS STEEL
How to define stainless steel ? Stainless steel gained its relevance due to a number of positive properties:
- Resistance to aggressive environmental influences;
- High wear resistance, due to which the service life of the products is more than ten years;
- Thermal resistance to sudden temperature changes;
- High level of resistance to corrosion and other types of destruction;
- Environmental Safety;
- Aesthetically attractive appearance;
- Easy to use and maintain.
Speaking about what stainless steel is and how to define stainless steel , we can say that it is the result of a successful mixture of steel and impurities that enhance its properties. Such impurities are the main component that prevents the formation of rust and premature aging of products. The more impurities, the longer the service life of the steel in general.
The main additional components are:
- Copper;
- Nickel;
- Molybdenum;
- Chromium;
- Manganese;
- Titanium.
Application of carbon steels
The mechanical properties of carbon steels are affected by the percentage of carbon. Based on this indicator, the following types of carbon steel are distinguished:
- low-carbon – carbon content up to 0.25%;
- medium carbon – 0.3-0.6%;
- high-carbon – 0.6-2%.
The amount of other elements (manganese, magnesium, silicon, etc.) in alloys is limited. There are no alloying components.
The main disadvantage of the material is its susceptibility to rust upon contact with air and moisture. At the same time, pipeline parts and shut-off valves are produced from carbon steel. In addition, smaller items: nails, wire, screws, gears, flywheels, crankshafts, drills, forging tools, etc.
The areas of application of alloys are regulated by regulatory documents. Ordinary quality steel - GOST 380-85; structural – GOST 380-88; instrumental - GOST 1435-54 and 5952-51.
The Art. brand is in demand in production. 20, high quality construction. It is used in construction and mechanical engineering, in the construction of pipeline systems.
- low price;
- good weldability;
- pliability to cold and hot processing;
- resistance to high temperatures.
TYPES OF STAINLESS STEEL
Based on the percentage composition of the main additives, it is customary to distinguish stainless steel from the following types:
Austenitic steels. They contain at least 20% chromium and 4.5% nickel.
Duplex steels. Their chromium content reaches 25%, 1.5% nickel and a slight admixture of nitrogen.
Ferritic steels. Up to 29% chromium is allowed in their composition.
Martensitic steels. Their chromium content is insignificant, no more than 13%, and nickel maximum 4%.
Multicomponent steels. Minimal amounts of chromium and nickel and include a wide range of other enhancing impurities.
Chrome is the main component that simplifies cold deformation, increases the service life of products, and gives an attractive appearance.
Despite the presence of a sufficient number of nuances, questions often arise: how to identify stainless steel, how to distinguish stainless steel from ordinary metal, and how to test stainless steel for quality components . To carry out a distinctive test in everyday conditions, when it is not possible to conduct a serious hardware examination, improvised means are used.
How to distinguish stainless steel from other types of steel
In appearance, all steel grades are almost identical, but at the same time they have different technical characteristics. This means that products made from different types of steel behave differently. To distinguish stainless steel from another grade of steel, you can use several methods:
- Use of nitric acid. This chemical liquid helps distinguish stainless steel from carbon steel. If you apply a few drops to the surface of the steel, a reaction will begin in which caustic steam will be released. This reaction is typical for carbon steel; no changes will occur with stainless steel.
- Check the reflection on the surface. Stainless steel has bluish-yellow tints on its surface.
- Marking. Steel products are always marked with the type and grade of material used. If the numbers are preceded by the letters “STAINLESS”, this means the use of alloy steel.
These simple tips will help you accurately determine the presence of stainless steel.
HOW TO IDENTIFY STAINLESS STEEL: 13 WAYS
Identifying stainless steel using a magnet
Stainless steel does not allow magnetization due to the action of Futko currents. But this technique does not always give the correct result, since iron and martensitic alloys have magnetic properties, and therefore, using a magnet, it is possible to reliably determine only austenitic-iron alloys, which contain a high percentage of nickel and chromium. In other words, it is impossible to 100% identify stainless steel with a magnet, but you can recognize its subtype.
Saline solution
The essence of the method of determining stainless steel with a saline solution is to identify susceptibility to corrosion. A strong saline solution serves as a good provocateur of corrosive destruction. For this purpose, the product to be checked is immersed in a saline solution for a day. Stainless steel, having a high degree of resistance to such aggressive environments, will remain undamaged by corrosion.
Slice method
An incision is made using improvised means. The color of the cut will help distinguish stainless steel from brass, which is similar in color. In the case of the latter, the cut will have a yellow tint. While stainless steel will remain light gray.
Determination of stainless steel with copper sulfate
The top layer is sanded using sandpaper. After which the surface of the stainless steel is treated with a solution of copper sulfate. In this case, the definition of stainless steel, as in the case of the above methods, the stainless steel will not change its external characteristics.
Physical method for determining stainless steel
How to check stainless steel physically?
The method is based on knowledge of the law on the volume of displaced fluid. Stainless steel placed in a container of water will displace a different amount of water than the metal can displace. To do this, you need to know the mass of the product, the mass of the displaced liquid, and have a table of weight differences on hand.
Marking
The marking indicates the properties characteristic of a given material. Based on these properties, you can understand how to identify and distinguish stainless steel from ordinary metal.
Blank Slate Method
Stainless steel does not leave marks from tight contact, while aluminum will give noticeable gray stripes.
Thermal conductivity
For aluminum, unlike stainless steel, it is much higher. In this regard, water in an aluminum container will boil much faster.
Aggressive environments.
When in contact with alkaline and acidic environments, the surface of stainless steel will remain unchanged. Spots will appear on the surface of the aluminum.
Reaction with nitric acid
A few drops of acid reacting with any carbon steel will cause the formation of corrosive fumes. Stainless steel will not react even if the surface is damaged.
Light ebb
The surface of stainless steel gives a yellowish-blue tint.
A mixture of hydrogen peroxide and 20% sulfide
Such a mixture applied to a cut will cause significant darkening visible to the eye if a non-ferrous metal has interacted with the reagent.
Hole
Drilling a hole will help distinguish stainless steel from duralumin by the appearance of the chips.
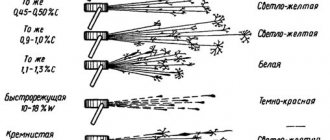
Identifying stainless steel using a spark
How to identify stainless steel using a spark? That's how:
- V (volume) of carbon in steel has a direct relationship with the number of flashes and sparks;
- The hue of the sparks gives information about the structure of the metal (if they are lightish white, then most likely it is steel with a low carbon content);
- If the sparks have a very bright light shine, this means that the material contains a large amount of titanium.
To conduct such a test to determine stainless steel, you need to start the process of grinding the material with a grinder, and sparks and flashes, as mentioned above, will provide the necessary information.
To carry out the test, an angle grinder (grinder) is required. Start grinding the surface of the steel and observe the reaction. The color, length and shape of the sparks will help you accurately determine metal or stainless steel.
A separate point is worth highlighting the differences between food-grade stainless steel and technical stainless steel. Due to the fact that cookware made from this type of steel is in high demand, such differences are quite relevant. The surface of food-grade stainless steel is distinguished by high quality processing, giving it smoothness. Even matte surfaces visually and to the touch do not have even the slightest defects or protrusions. As a rule, much more metal impurities are added to steel for this purpose. This is due to regular exposure to aggressive environments. The listed properties and requirements also apply to medical products.
In addition to all of the above, markings can provide a large portion of information about the steel from which the product is made.
Factors causing corrosion
There are a number of reasons why rust can form on a stainless steel product. Since there are hundreds of different alloys, it is important to consider that an environment that corrodes one type of steel may not affect another. We list several main factors leading to the development of rust:
Pitting corrosion
These are pinpoint, penetrating damage to the metal structure that occurs due to a violation of the surface layer. Pitting corrosion can develop on the surface of a material due to a lack of oxygen in a certain area. In this case, the zone where there is a lack of oxygen becomes anodic, and an excess becomes cathodic.
Many types of stainless steel alloys are susceptible to severe pitting when exposed to environments rich in chlorides (such as salt). Thus, grade 304 alloy, when used in marine applications, may begin to suffer from pitting as a result of contact with seawater or sea breezes enriched with salt. To avoid pitting, it is important to use stainless steel that is particularly resistant to chlorides, such as grade 316. Alternatively, the metal can be treated with a special coating to prevent direct contact with chlorides in the environment.
Bimetallic/galvanic corrosion
Bimetallic corrosion can occur when two dissimilar metals are welded together. Because when two metals with different properties are connected through a common electrolytic material, electric current can flow from one material to the other. This leads to the fact that the metal, which more easily accepts new electrons, will become an “anode” and begin to corrode faster.
The rate at which rust spreads will vary depending on the following factors:
- type of stainless steel being connected;
- type of electrolyte;
- ambient temperature and humidity, as well as the total surface area of the metals that are in contact with each other.
The best preventive measure against bimetallic corrosion is to apply a special coating to the metals that prevents the flow of electrons from the cathode to the anode.
It should also be noted that using a weld filler that is too different from the metals being joined can also lead to galvanic corrosion at the weld site.
"Transplant" of simple iron
In some cases, particulate matter residues from a plain steel or iron workpiece may be transferred to the surface of the stainless part. These particles can destroy the protective oxide layer of the workpiece, which subsequently leads to rusting.
The difference between this problem and the problem of bimetallic corrosion is that in this case the contact between dissimilar metals is purely accidental and usually occurs without the knowledge of the manufacturer. The most common reason why base metal particles end up on stainless steel workpieces is that equipment used to process one type of material may be used for another without proper cleaning between processes. To prevent particle transfer, it is important to thoroughly clean equipment when changing from metal to metal.
Intercrystalline corrosion
When steel is heated above the required temperature, a process of sensitization occurs - intergranular corrosion, leading to the precipitation of steel crystals from the surface of the material. Carbon atoms remove chlorine atoms from the alloy, which leads to a decrease in the percentage of chromium. When sensitized steels begin to come into contact with one or another aggressive environment, the grain boundary turns into an active anode, and the center of the crystal into a cathode. When intergranular bonds weaken, steel crystals fall out, leaving small pits with a black coating. If the temperature regime is observed, such problems do not arise.
Intergranular corrosion can also occur during welding. For ferritic alloys, the temperature leading to the appearance of rust is +900 degrees, for austenitic alloys +450 degrees. To prevent steel from rusting, weld zones are treated with special compounds. Passivation (the so-called processing process) is aimed at restoring the protective layer of metal at the welding sites. To do this, use various pastes and gels, citric or nitric acid.
Crevice corrosion
It develops in places where there are small gaps between the steel and another structural part. An example of this type of rusting can be the penetration of moisture under fasteners into the product. In this case, aggressive ions accumulate in the gap, which displace oxygen, which leads to the appearance of rust. Crevice corrosion can form between two joining surfaces - two metals or between a metal and a non-metal.
Erosive corrosion
Occurs due to the destruction of the oxide film by one or another abrasive. If stainless steel is regularly exposed to an abrasive liquid, its protective layer will be destroyed, which will lead to rust. To avoid this, it is important to avoid treating the surface of stainless steel products with abrasives. Chlorine has a particularly harmful effect on stainless steel, which is why chlorine-containing products should not be used when cleaning products.
STAINLESS STEEL MARKINGS
There are five types of basic markings:
- 08Х18Н10. Dishes made from such material are allowed for use in the food industry. However, exposure to caustic soda is not allowed.
- 08Х13. One of the most popular grades of steel, most often used in the manufacture of kitchen utensils. Such dishes can be heated to almost any temperature, and can also be stored in refrigerators and freezers.
- 20Х13-40Х13. This steel is used to make sinks and dishes. It copes well with temperature changes, is plastic and resistant to mechanical damage.
- 12X13. Steel products with this marking are used in the wine and alcohol industries.
- 08Х17. This steel has the highest heat resistance and good thermal conductivity. Pans made from this type of stainless steel are in high demand.
conclusions
Stainless steel is divided into several types depending on its composition - the more additives it contains, the higher its quality. All these standards are reflected by markings, according to which the material is classified into a certain group and used for the manufacture of various products. Stainless steel is mainly used in the food industry, in medicine, for the production of spare parts for machines and parts for construction.
You can check the quality of stainless steel yourself - visually, using chemical solutions or physical impact. All these methods are inaccurate and for a full assessment it is better to use professional tests. Purchasing material from a professional company guarantees the purchase of products of proven quality.
STAINLESS STEEL QUALITY ASSESSMENT
Assessment of the quality characteristics of stainless steel depends on various parameters, such as the amount of additives, method of connection, etc... After welding at the seams, stainless steel loses its resistance to corrosion, which can lead to the formation of rust and then to its destruction. Painted stainless steel will need to be cleaned of rust and re-polished, which will cause the steel to lose its resistance to moisture. To evaluate stainless steel in advance, you need to resort to a salt solution: if the material is of high quality, there will be no stains on the steel.
Distinctive features of galvanization and stainless steel
Stainless steel and galvanization differ in their production method, composition, durability, and these characteristics affect the difference in price, reaching 25–40%. This variation in cost is explained by the improved characteristics of corrosion-resistant stainless steel compared to galvanized metal.
Cink Steel
Galvanized steel is produced by coating a sheet of carbon steel with a thin layer of zinc, which over time (up to two years) forms a durable patina on the surface that is resistant to atmospheric moisture and oxygen.
RESULT: HOW TO IDENTIFY STAINLESS STEEL
Thus, the task of how to identify and distinguish stainless steel from any other types of metals and steels seems quite possible even without the use of serious industrial expert measures. It is enough to remember and apply at least some of the above methods, which provide completely objective information on the distinctive features. If doubts remain, it is better to turn to expert data. Especially when it comes to medical or food products. By the way, many people are also tormented by the question: is it possible to weld stainless steel to ferrous metal? In this article we will break everything down.
Stainless steel or carbon. Which knife steel is better? Choosing
Let's look at the characteristics of the two most common materials for knives - carbon steel and stainless steel.
The concept of carbon steel, in our particular case, combines medium and high carbon types. Of course, they are different and have different performance properties, but for our review today this is not significant. For those who like to argue and prove their point of view, let’s say right away that the article will not contain a final summary of which steel is better. You will be asked to use them for specific purposes.
I repeat once again - it is not correct to compare them - each job has its own good one.
General concepts
Once again, this opposition is a comparison between warm and round. When they say “carbon,” they mean its composition; “stainless” means the material’s ability to resist corrosion. Therefore, it is difficult to compare them.
Besides:
- According to the manufacturing technology , any steel
, and by the way stainless steel too,
contains
a certain amount
of carbon
a. It’s just that there is much more of it in the “carbon” one than in others. - Any
(yes any)
steel is subject to corrosion
. It’s just that for one it will require more time and certain harsh conditions, and some are covered with a film against water mist.
What determines the rate of metal corrosion: Corrosion is the oxidation of a material under the influence of oxygen. Carbon steel contains components that are more susceptible to this process. To slow down the process, metals (chromium, molybdenum), so-called additives, are added to the composition of stainless steel, which are practically not susceptible to oxidation.
It turns out that the main difference between one steel and another is the ability to resist corrosion. But there are others, more about them below.
Criteria for comparison
Odor absorption
Carbon steel, especially freshly sharpened steel, has a pleasant smell. But due to its porous structure, it strongly absorbs odors. This is normal as long as it smells like a forest, but if you use a knife in the kitchen, after some time the aroma will be more than unpleasant. To get rid of it, you will have to make a lot of effort.
Stainless steel, due to its structure, is less susceptible to absorbing odors, which makes it more desirable in the kitchen.
Sharpening and cutting
Corrosion-resistant steel contains a large number of additives to impart resistance to oxidation, wear resistance, etc. Therefore, sharpening and straightening such knives is quite complicated and requires special equipment.
Carbon steel, on the contrary, can be sharpened quite simply with ordinary musat and a thinner cutting edge can be made. This gives it good cutting properties. Therefore, according to this criterion, a knife made of carbon steel is superior to its colleague, but, unfortunately, not for a long time.
Care
For any knife to function properly, it must be periodically cleaned, trimmed and sharpened. You also need to take care of proper storage, away from cutlery and chemicals. But due to its composition, a device made of carbon steel is more capricious. A stainless steel knife is more unpretentious and does not require careful daily care.
Product appearance
Due to various processing methods and additives, carbon steel products have their own “individuality”. For example, blued steel has a noble black color; after blackwash coating, the blade acquires a greenish tint; various powder coatings give the coating a matte finish.
If desired, stainless steel products can be subjected to all these procedures, but, as a rule, this is not done.
Also, after some time, a thin dark film forms on the surface - a natural protection of carbon steel from corrosion. This also gives the blade an aesthetic appearance.
We anticipate your objections. We know that in the literature and on specialized forums there are different interpretations of all the evaluation criteria given in the article. And the opinions of those arguing are diametrically opposed.
Especially many copies were broken regarding sharpening and the ability to hold it on various blades. The fact is that pure steel, without additional processing, is practically not used for the manufacture of blades.
And these influences distort the overall picture.
By the way, the problem of corrosion of carbon steel is partially solved by treating it with an anticorrosive agent, but no treatment will improve the cutting properties of stainless steel.
Summary
With proper care, compliance with certain operating rules, timely editing and period, a carbon steel knife will solve most problems in the kitchen. Due to its good cutting properties and easy sharpening, it is more unique and convenient in household chores. And its appearance is pleasing to the eye.
If you want to get a simple, reliable multifunctional tool that can be used with equal efficiency both in the forest and in the kitchen, then opt for a stainless steel blade.
Here's some more interesting material on knives:
- TOP 5 best knives for hunting
- Damascus steel. Myths and reality
- Choosing steel for a knife
- TOP 5 incomprehensible and useless knives from domestic manufacturers
Other interesting articles:
- In the USA, bunkers are growing by leaps and bounds. Who builds them and why?
- How Alyoshka built an outpost in Chechnya.
- Devices for detecting enemy aircraft of the last century. What they were like.
- Photos of grandiose reconstructions of the Second World War
- Military exercises in the Soviet Union. chronicle: how it happened.
What is the difference between steel and brass?
Brass, unlike stainless steel, is obtained by fusing copper and zinc. ... However, grades with a high zinc content are rarely used. Two-component brass containing up to 97 percent copper is called red. Their second name is “tompak”.
Interesting materials:
What happens when you graft a pear onto an apple tree? How many apples can be harvested from 1 hectare of garden? How many kg of apples can you pick from an apple tree? How many kg from one apple tree? How many kilograms does one apple tree produce? How many varieties of apples are there in the world? How many varieties of apples are there in Russia? How many varieties of apples are there? How long to dry apples in the sun? In what month is it better to graft an apple tree?
Metals and alloys that are often confused
The silver alloy of iron and chromium is suitable for the production of kitchen utensils, medical instruments, bearings, cutting elements, etc. But these items are also made from the following materials:
- nickel-plated brass (a white copper alloy with a zinc content of more than 25%);
- cupronickel (silver-white metal made from an alloy of copper and nickel);
- white copper (an alloy containing at least 25% nickel).
Polished aluminum, nichrome, nickel silver and other alloys used for the production of cookware, knives, and jewelry can easily be confused with alloy steel. Despite their similar composition and high nickel content, they are easily distinguished at a scrap metal collection point and will not be accepted at the desired price. There are several ways to determine whether aluminum or stainless steel has fallen into your hands: chemical, mechanical, etc.
Types and grades of non-magnetic steels
If the origin of the product is known, the reaction with a magnet can roughly determine the type of stainless steel. The following brands are not magnetic:
- AISI 409 (analogue 08X13) - containers for cargo transportation, parts for the exhaust system of a car, etc. are made from this ferritic steel. (plasticity and lack of magnetic properties are due to the extremely low C content - less than 0.03%);
- AISI 304 (analogous to 8-12X18H10) - household items are made from it, as well as utensils and equipment for the food and pharmaceutical industries;
- 12Х21НБТ (ЭИ8П) – austenitic-ferritic steel for use in medium-aggressive environments, from which containers and equipment for the chemical and pharmaceutical industries are produced.
Stainless steel grades AISI 402–420, which contain from 11 to 14% chromium and less than 0.07% carbon, are not magnetic.