Bronze
Another common type of tin alloy is bronze, a tin-copper alloy. In principle, bronze also refers to copper alloys in combination with other elements. Any type of bronze contains small proportions of various additives (zinc, lead, phosphorus and other elements).
Mankind began to produce the well-known bronze back in the Bronze Age. It was used for quite a long period of time. It remained in demand during the Iron Age. It melts at 930–1140 °C. And the density of bronze is 7800-8700 kg/m3.
If initially arsenic bronze was in demand in the world, then with the development of horse-drawn transport and the foreign economy, tin bronze began to be used in a number of countries around the world. The use of this alloy in the rapidly developing sphere of large-scale industry was especially relevant. True, in recent decades it has begun to be replaced by non-tin varieties of bronze (aluminum, copper, etc.). It is believed that they are superior to tin alloy in their properties.
What is tin bronze? This is a copper-tin alloy, which contains more copper than tin. The positive properties of this alloy include the following:
- Hardness;
- Strength;
- Fusibility.
Tin bronze has these properties to a greater extent than pure copper. This alloy is resistant to sharpening and other types of processing. This suggests that it belongs to cast metals. Bronze has relatively low shrinkage. It is only 1% (for example, for brass and cast iron it is 1.5%, for steel it exceeds 2%). This allows the use of tin bronzes for the manufacture of castings.
Their advantages are such qualities as resistance to corrosion and excellent anti-friction properties. This explains the use of these alloys in the chemical industry. In particular, they are used for the production of cast fittings. Tin bronzes are no less popular in other industrial sectors.
Alloying components in these alloys are elements such as:
- Zinc;
- Nickel;
- Phosphorus;
- Lead;
- Arsenic.
And other metals. The zinc content in bronzes does not exceed 10%. Such a small content of this component does not in any way affect the quality of these alloys. At the same time, its use helps reduce the cost of producing tin bronzes and increases their resistance to corrosion. The addition of lead and phosphorus as alloying components has a positive effect on the antifriction properties of these alloys. In addition, this makes tin bronze easier to cut and press.
Their markings are presented as follows:
- Br OF 6.5-0.15;
- Br.OTs 4-3;
- Br.OTs10-2;
- Br.OF 10-1;
- Br.ONS 11-4-3.
Today these alloys are widely used in the transport industry.
Read also: Why do they put needles in the house?
The resistance of tin bronzes to rust and mechanical damage allows them to be used in the production of machine parts. Manufactured items are considered consumables, as they require regular replacement.
Bronze is durable. It is resistant to precipitation and mechanical stress. Products that perform a decorative function in theaters and palaces are also made from bronze.
Figure 3. Bronze products for oil and gas equipment
Alternative
It is worth saying that from the point of view of economy, tin-based babbits are very unprofitable, since this material costs quite a lot. In addition, tin itself is considered a scarce substance. For these two reasons, alternative bearings based on lead, antimony and copper were developed. In this composition, antimony crystals act as a solid base. The soft base is a direct alloy of lead and antimony. Copper is used here in the same way as lead in the previous composition, that is, to prevent the floating of solid base crystals.
However, it is also worth mentioning the disadvantages here. The lead-antimony eutectic is not as ductile as the tin phase. Therefore, parts made in this way suffer from rapid wear. To compensate for this disadvantage, you still have to add a certain amount of tin. The use of ternary eutectics is not very common.
Soon!
Home » Technical information » Articles » The main problems of pure tinOctober 12, 2007
Introduction
The growing attention of modern industry to issues of environmental conservation and concern for public health has recently greatly influenced the composition of the materials and technologies used in the production of electronics. In particular, lead-free soldering technology has become widespread. Lead is a material that causes significant harm to human health, but the refusal to use it in electronics has caused a number of technological problems. Newer lead-free solder alloys are known to have higher melting temperatures, which narrows the process window and thus results in more stringent controllability requirements for the soldering process. Some applications, such as PCB pad coatings and component leads, have begun to use pure tin due to its processability with the transition to lead-free technology. When using pure tin, a number of new problems also arise, primarily related to the properties of this material, which affect the reliability and performance of equipment in harsh conditions. In particular, tin is prone to the formation of filamentous growths - the so-called “tin whiskers” and is susceptible to “disease” in the cold - the so-called “tin plague”.
This article discusses the main problems that can arise when using pure tin instead of lead-containing alloys, their causes, and methods to combat potential defects.
Tin: characteristics and applications
Tin (lat. Stannum) is a chemical element located in the fifth period in group IVA of the periodic system of Mendeleev; atomic number 50, atomic mass 118.69; melting point 231.9°C, boiling point 2620°C, white shiny metal, heavy, soft and ductile. Tin is a rare trace element; tin ranks 47th in terms of abundance in the earth’s crust. It is used primarily as a safe, non-toxic, corrosion-resistant coating in its pure form or in alloys with other metals. The most important alloy of tin is bronze (with copper). Tin, in particular, is actively used to create superconducting wires based on the Nb3Sn compound.
Tin is widely used in the electronics industry as solder and coatings due to its good processability.
Pure tin as coatings
Coating of contact surfaces with pure tin is used to ensure solderability and protect the base metal from corrosion.
With the transition to lead-free technology, many manufacturers began to use pure tin to cover the leads and contact surfaces of components.
Coating of contact pads of printed circuit boards with immersion tin was previously used along with tin-lead coating, due to such a property as surface flatness, which is necessary for making high-quality solder joints. The flat surface of the contact pads coated with immersion tin allows for high-quality surface mounting of multi-lead components, including those with small lead pitches. In addition, the use of pure tin in lead-free technology ensures that there are no impurities of other materials introduced into the solder during soldering. These qualities, combined with a low price, have become a prerequisite for the widespread use of processes for applying immersion tin as a coating.
Immersion tin is chemically deposited onto the copper surface of the printed pattern through a displacement reaction. In this case, the metal of the coated base gives an electron to the tin ion in solution, which transforms into a metallic form, while the base metal dissolves anodicly:
Me0 + Sn2+ -> Me2+ + Sn0.
The standard electrode potential of copper is more positive relative to the potential of tin, so the substitution reaction can only occur in the presence of a complexing agent (thiourea), which shifts the potential to a more negative range of values relative to tin:
2Cu0 + Sn2+ + 4NH2CSNH2 + 2CH3S03H -> 2Cu (NH2CSNH2)2CH3S03 + Sn0 + 2H+[5],
where NH2CSNH2 is thiourea, CH3S03H is methane sulfonic acid [9].
The thickness of the tin immersion coating is about 1 micron.
However, with the beginning of the active use of pure tin with constantly growing requirements for microminiaturization of products, specialists were faced with new manifestations of the features of this material long known in metallurgy: the so-called. "whiskers" of tin and "tin plague".
Tin mustache
The formation of mustaches is a long-known phenomenon. It is characteristic not only of tin; metals such as zinc and cadmium are also prone to the formation of whiskers. In fact, the first published reports of tin whiskers date back to the 40-50s of the 20th century, but little attention was paid to this phenomenon in the electronics industry, since the growth of tin whiskers does not occur in the presence of a lead-containing coating of tin bases, as well as in the presence of a sufficient amount of lead impurity in tin. The use of the classic eutectic tin-lead alloy, which was most widely used before the transition to lead-free technology, ensured the absence of this problem.
Tin whiskers are thin threads that can grow vertically, bending, spiraling, in the form of hook-shaped or fork-shaped tin crystals. The length of the whiskers can reach 150 microns, which poses a serious risk of shorting adjacent elements of the conductive pattern of the printed circuit board. Whiskers, bending or tearing off during the manufacturing process of products and their operation, can form conductive bridges between current-carrying surfaces. In this case, at a sufficiently high current, the whiskers can melt, causing short-term failures. Whisker pieces can cause both intermittent and permanent product failures.
Rice. 1. An example of bending tin whiskers under a microscope. Photo from [1].
Rice. 2. An example of a tin whisker at 3000x magnification. Photo from [10].
It is impossible to accurately predict the formation of tin whiskers: they can appear both on new products and years after the start of use, both on the elements and under them. They may not appear at all. It is known that whiskers usually grow on coatings with a thickness of over 0.5 μm [9].
Until recently, there was no consensus among experts regarding the reasons for the growth of tin whiskers. Over the past few years, there have been significant advances in the study of whiskers and the main reasons for their formation, but, nevertheless, there is still no final consensus on the causes of this phenomenon. There are also no industry standards defining tin whiskers or regulating methods for combating them.
It has been established that the driving force in the formation of whiskers is the compressive stress in the tin layers. This stress can be a consequence of various reasons, such as the formation of an intermetallic structure, oxidation and corrosion, temperature cycling or mechanical stress [2].
In electroplated tin coatings, tensile stress occurs immediately after deposition, which weakens over time (3-5 days). After 5-7 days, the internal compressive stress begins to increase, which is a consequence of the formation of intermetallic compounds (Cu6Sn5 and Cu3Sn) at the boundary of the tin-copper layers, the molar volume of which is greater in relation to the volume of pure layers of tin and copper. As a result, a helical shear occurs along the grain boundary of the crystal lattice, where the growth of whisker crystals begins [9].
Immersion tin is thin, so no tensile stress occurs after plating. However, whisker growth still occurs, and the reason for their growth is the compressive stress resulting from the growth of the intermetallic layer. Since the thickness of tin is small, its atoms migrate along the boundaries between the metal grains to the place of growth of whisker crystals.
Thin layers of coating are most susceptible to internal stresses, since intermetallic compounds quickly absorb the layer of pure tin completely and oxidize. The optimal thickness of immersion tin, equal to ~1 μm, already poses a serious difficulty for the diffusion of intermetallic compounds [9].
Whiskers or dendrites?
Tin whiskers should not be confused with dendritic growth, which is also a relatively common cause of failure in electronic devices, resulting primarily in intermittent or permanent short circuits. The difference lies not only in the formation process, but also in what is known about the two phenomena.
Dendrites have been well studied because they are not a problem caused by the transition to lead-free technology. They are metal threads or crystals that grow on the surface of the metal (in the x-y plane), and not perpendicular to it (unlike whiskers), in the form of tree-like structures. The mechanism of dendritic growth is electrolytic in nature. That is, for dendrites to grow, it is necessary to have an electrolyte and voltage, and therefore, dendrites can lead to failures only if conditions for the formation of electrolyte are present (for example, humidity plus flux or organic acid residues), and also only during product operation.
Under the influence of the voltage present on the board, the anode conductor dissolves, releasing positively charged metal ions into the channel (Fig. 3a). The ions are directed through the channel to the conductor-cathode, where they are reduced to the metallic state, forming conductive bridges in the form of a dendrite-like loose metal structure in the insulating gap (Fig. 3b) [6]. The growth rate of dendrites at the cathode can reach 0.1 mm per minute [5]. As a result of these processes, whiskers with a thickness of 2...20 μm and a length of up to 12 mm can form in a few minutes (Fig. 3c). After the formation of a thread-like bridge, the crystals gradually thicken to 0.1 mm, acquiring a distinct metallic luster. The resistance of such crystals can reach up to 1 Ohm [6].
Rice. 3. Scheme of dendrite formation in a channel filled with ionogenic contaminants. Figure from [6].
The sequence of dendritic growth is clearly visible in photographs (Fig. 4).
Rice. 4. Stages of growth of metal dendrites: a - 2 min; b - 2.5 minutes; c - 3 min; g - 4 min. Photo from [6].
Dendritic growth is observed on conductors coated with Ag, Cu, SnPb, Au, and AuPd. To avoid the development of dendritic growth, manufacturers control the presence of moisture and chemical residues on the final products, which can dissolve the metal to form ions, which then form dendrites [1].
Intermetallic compounds in tin coating
As is known, Intermetallic compounds or Intermetallic compounds are compounds of two or more metals with each other. Intermetallic compounds refer to metal compounds, or metallides. They are formed as a result of the interaction of components during fusion, condensation from steam, as well as during reactions in the solid state due to mutual diffusion (during chemical-thermal treatment), during the decomposition of a supersaturated solid solution of one metal in another, as a result of intense plastic deformation during mechanical alloying ( mechanical activation) [7]. Essentially, an intermetallic compound is a thin boundary layer of interpenetration of soldered metals into each other.
In solder joints, the intermetallic layer plays the role of a mechanical bond. However, the formation of intermetallic compounds between the tin coating and the base material and their subsequent oxidation are a direct cause of deterioration in solderability. If the thickness of the tin coating is too thin, an ever-growing layer of intermetallic compounds absorbs pure tin, oxidizes and impairs solder wettability [9].
As already noted, the formation of intermetallic compounds may be the cause of the formation of tin whiskers.
The susceptibility of tin to the formation of intermetallic compounds is associated with its structure, which has a body-centered tetragonal crystal lattice. The aspect ratio of the lattice cell (s/a) is less than unity (rectangle in cross section). This non-cubic lattice structure indicates the anisotropic properties of the metal. For tin, the coefficient of thermal expansion and the coefficient of self-diffusion are greater in the direction of the longer side of the crystal cell.
Scientists have noted the unidirectional growth of tin whiskers [9], which is additional confirmation of the connection between the anisotropic structure of tin and the formation of whiskers.
In addition, due to the formation of intermetallic compounds, the appearance of so-called hairline cracks and the formation of weak solder joints is possible, which negatively affects the characteristics of the product.
White Tin "Disease"
The “disease” of white tin depends not so much on the combined use of tin with any other materials, but on its nature.
At the end of the last century, an interesting incident occurred: tin was sent from Holland to Moscow by rail. A train came out loaded with bars of white tin, but it brought only gray powder, useless for anything. On the way, tin “caught a cold” and was “attacked by a plague” [3]. This is one of several legendary stories where economic losses and even deaths occurred due to the tin plague.
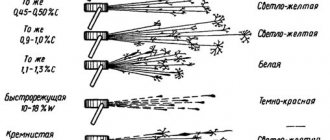
Rice. 5. Tin with 5% copper content after prolonged exposure at a temperature of -18°C. Photo from [4].
In reality, this “disease” is the result of a rearrangement of the order of atoms in crystalline tin.
Tin can come in two forms: the first is ordinary silver-white tin, a malleable metal that can also grow in the form of large single crystals. White tin is formed at temperatures exceeding +13.2°C. If the temperature drops below 13°C, then the tin atoms can rearrange and form crystals of another type - brittle non-metallic gray tin. The properties of these two types of tin differ significantly. The density of white tin is 7.3 g/cm3, and that of gray tin is 5.8 g/cm3. The temperature coefficient of volumetric expansion of gray tin is 4 times greater than that of white tin. Internal stresses that arise at the points of contact of different crystal lattices lead to the material cracking and crumbling into powder. The resulting modification already loses the properties of a metal and becomes a semiconductor.
It is known that both white and gray crystals are composed of the same tin atoms. However, the main reason for the difference is the arrangement of atoms in the crystal lattice. Changes in the size and shape of atomic structures completely change the properties of matter [3].
One modification changes to another the sooner the lower the ambient temperature. At a temperature of minus 33°C, the rate of this transformation reaches its maximum. If you pour boiling water over gray tin, then due to strong heating the atoms will rearrange again and the tin will turn back into the white variety.
Among metal physicists, the prevailing opinion is that the transition of white tin to gray begins with “contamination”: gray particles fall on the surface of white tin, and the mechanism of their action is similar to the action of the “seed” during the crystallization of liquids. However, there is an opinion that direct contact between white and gray tin is not necessary for infection with the “tin plague”.
Despite the fact that gray tin is a semiconductor in structure and type of connection between atoms, practically no practical applications for gray tin crystals have yet been found - they are too difficult to grow, they are fragile, and in terms of electrical properties they are no better than germanium and silicon, the industrial production of which fully mastered [3].
Methods to Prevent Tin-Related Defects
Currently, methods have been developed to combat the growth of intermetallic compounds, the appearance of tin whiskers and tin plague, thanks to which the likelihood of their occurrence can be avoided or reduced.
It has been found that various conformal coating materials can help reduce damage caused by tin whiskers. Coatings do not prevent whisker growth, but studies have shown that some coatings slow or inhibit whisker formation. In some cases, the resulting whiskers are “locked” inside the coating, which prevents their development, leading to short circuits [2].
The use of coverings that are not thick or strong enough to control whisker growth is controversial. Coverings that have micro-perforations are generally useless as they allow moisture to penetrate. This moisture creates the conditions for potential dendritic growth and also provides a conduit for whisker formation. Tin whiskers are very stable. They will grow under the covering and, if it is not strong enough, small tendrils may grow through it [1].
In addition, corrosion could theoretically be a significant source of compressive stress in tin films and, as a consequence, could cause whisker growth. Therefore, measures must be taken to prevent severe oxidation and moisture condensation.
The iNEMI Tin Whisker User Group's primary recommendation for whisker suppression is the use of a nickel interlayer between the tin plating and the copper base. Parameters such as thickness, porosity and elasticity of the nickel coating are very important to provide an effective barrier layer for copper [2]. Moreover, due to the creation of such a layer, the diffusion of copper and the formation of tin intermetallic compounds are limited. Plating nickel onto a steel substrate has also been found to be effective.
It is recommended to avoid applying tin over brass as this combination of metals tends to create whiskers. Tin plating of brass can only be used when a nickel diffusion barrier is applied. The minimum thickness of a nickel diffusion barrier is 1.27 µm [2].
If the coating is exposed to prolonged mechanical pressure, the risk of tin whisker growth increases significantly. Careful testing must be carried out to determine whether the growth of whiskers will reduce the reliability of the product.
Tin plague is a fairly rare phenomenon in the electronics industry. Even if coatings of completely pure metal tin are used, after soldering it dissolves in the solder alloy, and in the presence of impurities, tin is no longer susceptible to tin plague. That is why tin is used for soldering and the soldered products do not fall apart. As a rule, absolutely pure tin is not used in coatings of component leads; impurities are necessarily added to it, even a small amount of which can eliminate this problem. If you add a little, for example, bismuth to tin, you can prevent tin plague. Bismuth atoms in the crystal lattice of tin interfere with the restructuring, and white tin remains a metal and does not collapse even at low temperatures. In addition, alloying tin with antimony, cobalt and other metals became a remedy against the tin plague. It was found that aluminum and zinc, on the contrary, contribute to the process of plague formation.
Some manufacturers limit the shelf life of pure tin plated components at lower temperatures. The tin plague effect should also be considered when using solders with high tin content. Since the “tin plague” has a strong effect only at temperatures below -40°C (at near-zero temperatures the transformation process takes many years), its effect on lead-free components is currently poorly studied.
Conclusion
Despite these advances, it is still clear that we do not fully understand the basics of tin whisker formation and the process by which they grow. There are no quantitative models that can predict and forecast whisker growth. The iNEMI Tin Whisker User Group has developed basic methods and standards aimed at reducing the compressive stress in tin films and thereby preventing the formation of whiskers. All of these recommendations are based on empirical data. And if today there are proven methods for preventing the tin plague, it is still impossible to guarantee the complete absence of mustaches after the process of applying tin.
List of sources used
- Advanced coating technologies for lead-free soldiers, Bill Boyd, Specialty Coating Systems, Indianapolis, IN, USA www.globalsmt.net
- iNEMI Updates Tin Whisker Recommendations, Joe Smetana www.globalsmt.net
- About the tin plague and scientific foresight.gornie-porodi.info
- Tin Plague, Andrew D. Kostic, Ph. D. Senior Consultant, Willcor klabs.org
- Technology recommendations about www.spring-e.ru
- Arkady Medvedev. Assembly fluxes. To rinse or not to rinse? “Components and Technologies”, No. 4, 2001 www.compitech.ru
- Intermetallic compounds. www.xumuk.ru
- JSC "OSTEK Enterprise" Encyclopedia of surface mounting. Lead test. Do the components you use contain lead? www.ostec-smt.ru
- Immersion tin as a finishing coating. Reliability comes first! "Technologies in the electronic industry", No. 4, 2007
- Whisker Evaluation of Tin-Plated Logic Component Leads. Douglas W. Romm, Donald C. Abbott, Stu Grenney, and Muhammad Khan. Texas Instruments. Application Report SZZA037A - February 2003.focus.ti.com
all articles
Conditions for scrap delivery
The material that is brought to the scrap metal collection point must meet certain requirements:
- Be clean. This implies cleansing from physical dirt.
- Sorted. This way you can earn more money from selling scrap.
- Meet standards. Otherwise, it will take time to determine the percentage of various metals in a particular scrap.
Proper preparation can increase the price per 1 kg of scrap. If there is no time to do this, then the optimal solution is to transfer the scrap metal to an intermediary organization, which will take care of all the worries for a small fee.
How to determine tin: basic methods
Tin is often mixed with the cheaper lead, so you need to know exactly how to distinguish one metal from the other. In particular, tin “crunches”, so it is enough to melt it into a rod, bring it to your ear and bend it (there should be a characteristic crunch).
Here are the main differences between these two metals:
- Lead is heavier than tin. It is enough to take two pieces of metal in your hands, and you can accurately determine which material is which.
- Tin has a silvery-white tint, while lead is darker in color.
- Tin bends easier and is more pliable.
- The most basic way is to take a soldering iron and try to melt the metal (the tin will immediately “float”).
Methods for determining tin
- Method number 1. Lead weighs more than tin. If you take one metal in both hands, one of which is tin, then you can accurately identify it.
- Method number 2. The appearance of metals also has its differences. While pure tin has a pleasant whitish-silver tint, lead is much darker. In addition, lead reacts much more actively in the oxidation reaction in air.
- Method No. 3. Tin is a low-melting, brittle metal that can be deformed under any influence.
- Method number 4. It has long been known that pure tin has the property of crunching when bent. Naturally, lead is not endowed with such characteristics.
- Method No. 5. Lead has a much softer structure than tin, so the former metal can be cut with an ordinary knife or simply bent.
- Method number 6. To check the authenticity of the material, you can use a traditional soldering iron. Tin melts at high temperatures. If you touch it with a well-heated tool, real tin will float.
Why do you need to determine tin?
Each of the above methods has its pros and cons. Experience shows that it is quite difficult to make a mistake in choosing genuine tin. It can only be confused with silver. And even then, only because of the similar appearance. As mentioned earlier, tin is painted in a light silver color, which makes it similar to the traditional valuable metal - silver.
As a rule, people want to know how to identify tin and what metal is in front of them when they find metal rods of unknown origin among the trash in their garage. This topic is often discussed on specialized forums on the Internet. There is probably no clear answer to the question of which method is the most effective. Therefore, a person can try each of them and independently choose the best one.
How is tin obtained from tin powder?
How to lighten tin?
Products made of tin and other areas of its application
Alloys
In the modern national economy, in the vast majority of cases, it is not tin that is used, but its various alloys.
- The most ancient and well-known area of use is the production of tin bronze , that is, an alloy of copper and tin. It has not only excellent aesthetic qualities, but excellent technical ones: bronze is durable, hard, wear-resistant, not susceptible to corrosion, and so on. Well, the beauty of the alloy was appreciated a very, very long time ago: bronze sculptures, dishes, and household items still attract people with their richness of color and shine.
Modern bronze often includes more than just copper and tin. Usually it also contains silicon, lead, aluminum and other additional components.
- The second most famous application is solders . These are alloys of tin and lead, silver, copper, as well as cadmium or bismuth. A distinctive feature of this alloy is its low melting point, the ability to form bonds with other metals and the high strength of such compounds. With the help of solders, a wide variety of metal parts are connected to each other, which cannot be connected to each other - due to too different melting temperatures, for example. Occasionally, pure tin solders are also used.
The properties of solder are determined by its composition. Traditionally it is used in radio and electrical engineering. But an alloy of 30% tin and 70% lead has a very wide range of solidification. This characteristic is used when soldering various types of pipes.
- Both tin itself and tin-lead alloys have good adhesion to the metal. And therefore, both are used for external coating of parts in order to protect products from corrosion and give them an attractive appearance. A layer is applied by immersing the object in a bath of melt, or using the electrolytic method from aqueous solutions.
- Another well-known alloy of tin, antimony and copper is known for its outstanding anti-friction properties. Such compositions, called babbitts, are used to coat various moving parts in order to reduce their wear.
- An alloy of metal with lead and antimony is used in the manufacture of typographic fonts. Its durability and resistance to fatigue allow the same set to be used for a long time.
- Another unusual use for combining metal with lead is in organ pipes. Tin is the most tonally resonant metal known. Its amount in the alloy determines the tone of the pipe.
This video will tell you about the uses of tin:
Independent substance
Tin is also used as a supply of an independent substance - with a share of up to 97–99%.
- Almost half of such pure metal as tin is used to coat cans. Well-known tin objects are a steel product coated with a thin layer of tin - 0.4 microns. The latter provides excellent anti-corrosion protection.
- A lot of different food containers and even dishes are made from tin, since the metal has excellent hygienic properties and is absolutely safe, unlike its medieval “brother”, which is an alloy with lead. Dishes made from this light silver metal are very beautiful. In addition, the high malleability and plasticity of the substance make it possible not only to stamp pots and plates, but to produce truly excellent tableware items. Accordingly, gifts made of tin are popular.
- Due to its excellent anti-corrosion properties, tin is also used in the manufacture of pipelines. These qualities are especially valuable when organizing a drinking water supply system. However, they are not widely used, since the material is quite expensive and, most importantly, scarce on the construction market.
We will talk about the heat, degree, specific melting temperature of tin for making products and soldering microcircuits, about the features of the use in industry of white, gray, chlorine, liquid tin, and its properties below.
How is tin obtained?
To get tin
varietal grades of the order of Sn 96.5 - 99.9%, fire or even electrolytic refining is used.
And tin
with a purity of several nines - Sn 99.999%, which is in demand mainly in the semiconductor industry,
is obtained
by the method of zone melting.
Interesting materials:
In what year was television invented? In what year was color television invented? In what year did people watch television for the first time? What year did the camera appear? In what year did sound recording appear? In what year did the voice recorder appear? In what year did gramophones appear? In what year did scissors appear? In what year did the first suspended ceilings appear? In what year did the first flat-screen TVs appear?
Scrap Tin Cost
At our collection points, the most valued pure metal is tin, marked O1 and O2.
Most often they bring different types of solder. Their cost depends on the tin content in them (the following are types of solder, in which the number is the percentage of tin content in the composition):
- POS-90. Used in jewelry production, it has the highest metal content and, as a result, a higher price.
- POS-61. Used for installation of radio engineering parts.
- POS-50. Used for soldering parts for which increased strength is important.
- POS-40. Solder for soldering conductive metal products.
- POS-30. Highest lead content (70%). Used for soldering steel parts.
Scrap from cans and tin is considered the least valuable. This non-ferrous metal is also found in bronze, but bronze scrap is assessed as a separate category.
accepts various types of tin scrap in strict accordance with current legislation. Payment is possible immediately after weighing the scrap metal. We work officially, so we provide all the necessary documents for government services.
Soldering process
Combining 2 or more parts using soldering is carried out for:
– obtaining electrical contact with low resistance;
– obtaining a strong seam (sometimes thermal, absolutely sealed).
The soldering process is based on the difference in melting temperatures between the solder and the metals being joined. While the soft alloy melts and becomes liquid and fluid, the metals being joined remain solid. Molten metal flows over the parts being joined, filling the voids between them. During the soldering process, an intermediate layer is formed, which includes the combined parts of the solder and the material of the elements being combined. With its help, a single structure is formed from two or more parts. Rosin or an alcohol solution of rosin act as a flux when soldering PICs.
Before starting work, you need to choose the right solder based on:
– properties of the combined materials;
– requirements for connection strength;
– corrosion resistance of the junction;
And when soldering parts that conduct current, the conductivity coefficient is also taken into account.
For soldering copper wires, use POS-40 based on rosin. Stainless steel can be joined with conventional POS, but for flux they use a special material that is more active than rosin.
Where is tin mined in Russia?
According to Dalnedra, in the territory of Yakutia, Primorye and the Khabarovsk Territory and Chukotka there are about 95% of proven reserves of Russian tin
. The largest facilities with reserves of more than 100 thousand tons are located in Eastern Yakutia, Chukotka and Khabarovsk Territory.
Interesting materials:
How to undo recent changes to the registry? How to undo all changes in Photoshop? How to edit on a chart to change the chart type? How can I see the latest changes in a folder? How to use a mask to change color in After Effects? How to save a change in an element's code? How to save changes to hosts? How to save changes in the site's html code? How to save changes in developer tools? How to save changes to your Instagram profile?
Gems: Guide
Today, tin is a common metal. However, in centuries it was quite rare and valuable, so Russia bought it from other countries. Tin was used to coat iron products to protect them from rust. From this metal, Russian craftsmen created dishes with relief patterns, since soft, pliable tin could easily be processed with a chisel. After casting the product, the master decorated it with intricate patterns or an engraved inscription. Today, the Moscow Historical Museum has various examples of tin utensils that have been preserved from ancient times.
How much does tin cost?
The average cost of 1 kg of tin ranges from 700 to 900 rubles.
Interesting materials:
What paint should I paint the flowers with? Which foot to sew velvet with? What is the best thermos for tea? What is the maximum period for which a hemostatic tourniquet can be applied? What is the maximum prison term for women? Which maniac will be released on March 3? What marker does not wash off from fabric? Which continent is washed by the Atlantic and Pacific Oceans? What month is Traven in Russian? What metal is in a tin can?