Stanislav Virbilis
NICKEL PLATING Properties and application of nickel coatings Nickel plating is the most popular electroplating process.
Attractive appearance, high corrosion resistance and mechanical properties speak in favor of the wider use of nickel for decorative, protective and functional purposes. Nickel applied directly to steel is in the nature of a cathodic coating and therefore protects only mechanically. The discontinuity of the coating contributes to the formation of corrosive couples, in which steel is a soluble electrode. This results in corrosion under the plating, which erodes the steel substrate and causes the nickel plating to peel off. In order to prevent this phenomenon, the steel must be coated with a dense, non-porous, thick layer of nickel.
Nickel coatings are the basis of multilayer decorative and protective systems Ni-Cr and Cu-Ni-Cr. The use of baths with shine formers greatly simplifies the technology of applying multilayer coatings. Due to its high mechanical properties, nickel is used to restore worn machine parts, galvanoplastic production of injection molds, and structural elements that are difficult or even impossible to produce using conventional mechanical methods. In the chemical industry, a thick layer of nickel is used to cover parts exposed to strong alkalis.
Increased service life of nickel plating
Nickel plating can be subject to continuous surface corrosion. The corrosion process appears only in the initial period. As the temperature of the working solution increases, surface corrosion penetrates deep into the material. Then this process slows down and stops completely.
Copper plating of the key
To increase the service life of the nickel coating, copper plating technology is used. Copper plating can also eliminate minor surface defects. The application of copper as a substrate ensures the reliability and durability of nickel protection.
The porosity of the copper coating causes the destruction of the protective layer and reduces the service life of the finished product. The substrate metal undergoes corrosion, followed by peeling of the protective layer.
Most often, products with a single-layer protective coating are exposed to corrosion processes. Multilayer parts are exposed to harmful factors to a lesser extent.
To protect products from damage, a number of additional measures are taken. Special additives are used to close the pores.
For your information. To prevent loss of hardness, nickel plating of steel is carried out at a temperature of 250-300ºС.
Additional processing of parts to extend service life
Nickel plating at home is carried out using the following methods:
- The dry magnesium oxide reagent is mixed with water until it turns into a paste. The resulting mass is carefully processed and the part is immersed for several minutes in 50% hydrochloric or sulfuric acid.
- The working surface is wiped with penetrating lubricant. The product is then immersed in purified fish oil. Excess fat is removed after 24 hours using gasoline or other solvents.
- Larger parts are treated with fish oil in two passes. The interval between treatments should be at least 12 hours. After two days, excess slave fat is removed.
Installation diagram for nickel plating at home
The use of nickel alloys with other metals can improve the physical and chemical properties of nickel.
Aluminum helps increase the electrical resistance and corrosion resistance of nickel.
Tungsten, molybdenum and titanium increase its heat resistance.
The addition of chromium increases the resistance of the nickel coating in oxidizing and reducing solutions.
Copper increases nickel's resistance to various acids.
Nickel plating baths
Widely used in workshops is a bath consisting of three main components: nickel sulfate, boric acid and chloride, such as nickel.
Nickel sulfate is a source of nickel ions. Nickel chloride significantly affects the performance of nickel anodes. In chloride-free baths, strong passivation of nickel occurs, as a result of which the nickel content in the bath decreases, and the result is a decrease in current efficiency and deterioration in the quality of coatings. In the presence of chlorides, the anodes dissolve to a degree sufficient for normal nickel plating. Chlorides increase the conductivity of the bath and the “operability” of the bath when contaminated with zinc. Boric acid makes it easier to maintain pH at the same level. The effectiveness of this action largely depends on the concentration of boric acid (in practice, predominantly ~30 g/l).
The use of nickel chloride is not considered desirable by everyone. Due to its high price, and often difficulty of acquisition. However, it is necessary to take into account that when introducing nickel chloride into the bath, we also introduce nickel into the bath. NiCl2•6H2O chloride theoretically contains 24.69% nickel and, therefore, by introducing this chloride in an amount of 40 g/l, we increase the nickel content in the bath by approximately 10 g/l, which already has a certain value.
Another frequently debated issue is the use of sodium chloride as a source of chlorides. It is known that many electroplating shops successfully introduce NaCl into bright nickel plating baths. There are many reasons for poor performance of nickel baths, and the claim that the reason is the use of table salt has little justification. Even the English company Canning, well known among electroplating technicians, introduces sodium chloride in the amount of 28 g/l into the NiSOL bright nickel plating bath. Instead of sodium chloride, another chloride can be used, for example, as in PNR, magnesium chloride. The Watts sulphate bath has been known and widely used for many years. The contents of individual components - the basis of Watts baths for applying matte coatings are, g/l: (200-350) NiS04•7H20, (30-60) NiС12•6H20, (25-40) H3BO3.
Often, additives of so-called electrically conductive salts are added to the Watts bath, which increase the electrical conductivity of the bath and improve the appearance of the coating. Among these salts, the most commonly used is magnesium sulfate (~30 g/l); in baths for mass treatment of small parts, its concentration is much higher.
Nickel sulfate is most often introduced in a concentration of 250-350 g/l. For a long time, an upper concentration limit was considered appropriate, which made it possible to carry out the process at high current densities without fear of burning the coating on the ribs and protruding areas. Recently, there has been a tendency to limit nickel sulfate to less than 200 g/l, which significantly reduces solution losses.
The concentration of chlorides in a nickel bath is not precisely standardized. In so-called chloride baths the concentration of nickel chloride exceeds 200 g/l and therefore there is no need to add nickel sulfate. In a workshop environment this is not justified, even based on the price of nickel chloride.
The concentration of boric acid reaches 25-40 g/l. Below 25 g/l the tendency for the nickel bath to become alkalized rapidly increases. However, exceeding a level of 40 g/l may be unfavorable due to the possibility of boric acid crystallizing in the form of crystals deposited on the walls of the bath and anodes. This phenomenon occurs especially easily in unfavorable or poorly heated baths.
The sulfate bath operates over a wide range of temperatures, current densities and pH. At room temperature, nickel plating is rarely used. For coatings applied in cold baths, nickel peeling off along with the chromium often occurs during decorative chrome plating. Therefore, the bath should be heated to at least 30 °C.
The bath with blackening agents operates at 50-60 °C. The current density must be selected experimentally so that coatings are not burned. The sulfate bath works reliably over a wide pH range. Previously, baths were usually maintained at a pH value of 5.4–5.8, which was motivated by the less aggressiveness and better hiding power of the bath. However, such a high pH value leads to a significant increase in stress in nickel coatings. Therefore, in most baths used in industry, pH = 3.5–4.5. Modern baths require mixing, which is necessary from the point of view of intensifying the nickel plating process and reducing the risk of pitting. Stirring the bath entails the need for continuous filtration in order to eliminate mechanical impurities. Mixing with a moving cathode rod is not as effective as mixing with compressed air, and in addition, it requires a special ingredient that prevents foaming. Accordingly, purified air is currently used in the galvanic workshops of Poland to mix all nickel baths equipped with devices for bright nickel plating, manufactured at the Institute of Precision Mechanics.
Electrolytes for application.
For nickel plating, sulfate, chloride, sulfamine, borofluoride, oxalate and other electrolytes are used, in which nickel is in the form of a divalent cation. A large number of compositions and deposition modes have been developed that make it possible to obtain nickel deposits with various physical and chemical properties.
Watts' sulfate electrolyte is most often used, since the substances it contains are the most accessible, and it is easy to prepare and maintain.
The main component of the sulfate electrolyte is nickel sulfate NiSO4•7H2O. Technical nickel sulfate grade CH-1 is green crystals. Solubility without heating reaches 300 g/l.
In addition to nickel salts, which are sources of nickel cations, the electrolyte contains components designed to increase electrical conductivity, stabilize acidity (buffer additives), improve the solubility of anodes (chlorides), add shine to deposits, and prevent various defects encountered during nickel plating.
If the concentration of NiSO4•7H2O does not exceed 300 g/l, Na2SO4•10H2O and MgSO4•7H2O are sometimes added to the electrolyte to increase electrical conductivity. Sodium sulfate has significantly greater electrical conductivity, but magnesium is included in nickel coatings, making them softer and lighter.
Boric acid is the most widely used buffer compound. Boric acid regulates the pH not only in the total volume of the electrolyte, but also in the near-cathode layer, in which the pH level continuously increases due to discharge and hydrogen release. At pH>4, precipitation occurs through the film of the resulting nickel hydroxide. For low pH electrolytes, fluoride additives are more effective.
General characteristics of bright nickel-plated baths.
Traditional baths for applying matte coatings are currently used indefinitely. They are used, in particular, for pre-nickel plating of steel products before acid copper plating, in the belief that on matte nickel the matte coating deposited in an acid bath has better adhesion than on bright nickel. This assumption is sometimes justified, since decomposition products of organic additives accumulate in baths with brightening agents, leading to passivation of nickel coatings, mainly in galvanic workshops, whose workers neglect the rules for preserving nickel baths that have been used for a long time without regeneration. However, there is a situation that forces one to abandon nickel plating in baths with shine-forming additives, for the reason that shiny coatings are not flexible enough and are destroyed when nickel-plated objects are bent. Before deciding to use bright nickel plating in series production, the suitability of the process must first be tested on samples. Consumption of nickel anodes. Practitioners who deal with nickel plating on a daily basis know that nickel consumption is associated mainly with the consumption of nickel anodes, which also determines the need for frequent replenishment of the anodes; At the same time, the bath works for months without the need to add nickel sulfate. Wanting to save on nickel, some workshop workers take the line of least resistance and simply apply a thin coating, thereby degrading the quality of the product. The dependence of the mass of deposited nickel on the coating thickness is shown in table. 5. TABLE 5 TIME REQUIRED TO OBTAIN A NICKEL COATING OF A GIVEN THICKNESS, S, AND THE MASS OF DEPOSITED NICKEL, mNi, DEPENDING ON THE CURRENT DENSITY. CATHODE CURRENT OUTPUT 92.5%
| s, µm | mNi, g/dm2 | t, min, at J, A/dm2 | ||||
| 1 | 2 | 3 | 4 | 5 | ||
| 2,0 | 0,181 | 9,3 | 4,7 | 3,1 | 2,3 | 1,9 |
| 5,0 | 0,453 | 26,5 | 13,2 | 8,9 | 6,6 | 5,1 |
| 7,5 | 0,680 | 39,7 | 19,8 | 13,3 | 9,9 | 7,9 |
| 10,0 | 0,890 | 52,8 | 26,4 | 17,6 | 13,1 | 10,6 |
| 15,0 | 1,330 | 79,5 | 39,7 | 26,5 | 19,8 | 15,8 |
| 20,0 | 1,780 | 105,8 | 52,8 | 35,3 | 26,4 | 21,2 |
| 25,0 | 2,230 | 130,0 | 66,1 | 43,1, | 32,9 | 26,4 |
| 30,0 | 2,670 | 155,0 | 77,5 | 51,6 | 38,75 | 31,0 |
| 40,0 | 3,560 | 205,0 | 102,5 | 68,3 | 51,25 | 41,0 |
| 50,0 | 4,500 | 262,0 | 131,0 | 87,0 | 65,5 | 52,4 |
Thus, with a nickel coating thickness of 10 microns, often used as a sublayer under decorative chrome for light operating conditions, 0.89 g of nickel is theoretically deposited per 1 dm2 of surface, but in practice it will be more. Even if we accept a nickel consumption of 2 g/dm2 and assume that 1 kg of nickel costs 1000 zł, it turns out that the cost of the required nickel is 2 zł. Consequently, reducing the thickness of the coating does not provide much savings and can damage the company's reputation.
General information about chemical nickel plating (Ni-P, Chemical N).
Externally, the Ni-P coating has a yellowish-white color and a slight shine. The presence of phosphorus in the coating leads to a noticeable deviation in the properties of the coating from pure nickel. Thus, the coating density, depending on the phosphorus content in the alloy, ranges from 7.9 to 8.2 g/cm3. In terms of electrical conductivity and magnetic characteristics, the Ni-P alloy is inferior to pure Ni, the more the higher the concentration of P in it. The coatings have minimal porosity and high decorative properties (especially when deposited from a freshly prepared solution), therefore they are used as protective and decorative.
The electrochemical nickel plating process makes it possible to deposit coatings of uniform thickness with deviations of no more than 10% on parts of complex configurations. Compared to nickel coatings obtained by electroplating, they have higher hardness and wear resistance, so they can be used for parts operating under friction conditions, especially in the absence of lubrication.
| Designation | Chem.N Ni-P ENP (Electroless Nickel-Phosphorus) |
| Thickness | 6-50 microns (greater thickness is also possible) |
| Microhardness | 6400 MPa 11000 MPa - in case of heat treatment of the coating |
| Electrical resistivity at 18°C | 6.8-10-7 Ohm⋅m |
| Permissible operating temperature | 650oC |
| Light reflectance | 75% |
| Phosphorus content in Ni-P alloy | 0-4% (crystalline coatings), 4-8% (have 2 phases: crystalline and amorphous), 8-14% (amorphous coatings) |
High protective properties along with low porosity make it possible to use nickel-phosphorus coatings as protective coatings, including in conditions of superheated steam and air, up to 700 °C. To increase wear resistance and reduce the coefficient of friction, a nickel-phosphorus coating is applied to the rubbing surfaces. The coating is indispensable in the field and in small workshops for restoring the dimensions of worn parts. It is advisable to coat large parts.
The adhesion of nickel-phosphorus coatings is stronger than the adhesion of electrolytic nickel, since deposition occurs evenly both inside and outside the part, filling all micro-cavities and irregularities. Thickness deviations do not exceed 10%, so chemical nickel is applied to precision parts, for example, plunger pairs of engine fuel pumps, small parts in the watch and optical industries, etc.
The disadvantage of the coating is its fragility, which begins to appear at a layer thickness of about 10 microns and above.
Electroless nickel plating is an autocatalytic topochemical process.
The nickel reduction reaction is autocatalytic, i.e. To start it, a catalyst must be present on the surface to be coated. Catalytic properties are usually possessed by the base metal, for example iron, titanium, aluminum, and then by the nickel coating itself (hence the name “autocatalytic”, i.e. nickel itself provokes its growth on the part being coated).
Chemical nickel can also be applied to those metals that are not catalysts for the reduction reaction: copper, silver, etc. In this case, preliminary contact of the part with a more negative metal, for example aluminum, or the application of a short current pulse is necessary.
It is impossible to obtain chemical nickel coating on lead, cadmium, and tin.
Chemical nickel is also applied to non-metallic materials: glass, ceramics and plastic. Before coating, the surface is subjected to activation using known methods.
Preparation of nickel plating baths.
To prepare nickel plating baths, it is recommended to use demineralized water or, in extreme cases, tap water. Ground water should not be used. Working and spare baths made of steel sheet are lined from the inside with hard rubber or polyvinyl chloride. Both baths are thoroughly washed and filled with water, adding sulfuric acid ~5 g/l.
The next day, the baths are thoroughly rinsed and the spare bath is filled halfway with water. The water is heated to 60 °C and with constant stirring, first boric acid is dissolved, then nickel sulfate and nickel chloride.
Since technical chemicals contaminated with foreign metals and organic compounds are usually used to prepare a bath, preliminary cleaning of the bath is necessary. For this purpose, the pH of the bath is increased to 5.0 by introducing freshly precipitated nickel carbonate obtained from nickel sulfate. In a separate vessel, half filled with warm water, dissolve nickel sulfate and fill it with an aqueous solution of sodium carbonate until the green precipitate of nickel carbonate completely disappears. Carefully drain the water, and add the remaining sediment to the bath in small portions with constant stirring until pH = 5.0. Commercially available basic nickel carbonate is not as good as freshly prepared nickel carbonate because it is less soluble.
Some simplify the process of increasing pH by adding 20% NaOH in small portions to the bath instead of nickel carbonate. The solution must be vigorously stirred for an hour until the green suspension of nickel carbonate is completely dissolved.
After increasing the pH to 5.0, add an aqueous solution of potassium permanganate in small portions with constant stirring until a stable pale pink color appears. Then add activated carbon “Carborol S-extga” (1 g/l) and stir for 2 hours. The bath is left alone until the next day. In this state, the bath should have the natural color of nickel sulfate, but if it does not have this color, then add sulfuric acid to pH = 3.8-4.0; onto the cathode rod until the purple color disappears.
Further cleaning consists of running the baths at low current densities. The solution, previously purified in the spare bath, is pumped into the working bath, adjusted with demineralized water to the specified level, and sulfuric acid is added to pH = 3.8–4.0; the maximum possible number of corrugated steel plates is hung on the cathode rod and a current with a density of initially ~0.5 A/dm2 is turned on, and after several tens of minutes it is reduced to 0.2–0.3 A/dm2. Electrolysis lasts 6 hours with constant stirring and temperature ~ 60 °C. Once the pH is adjusted to normal, the cleaned bath is ready for use. Test loads are nickel-plated in it in order to select optimal processing conditions. For bright nickel plating, appropriate substances are added. Operation and regeneration of nickel baths. Stabilization of nickel plating baths consists of maintaining specified concentrations of individual components and regulatory removal of contaminants. It is easiest to adjust the composition based on chemical analysis, but an experienced electroplater can solve these problems independently.
Particular attention should be paid to monitoring and adjusting the pH - a daily responsibility of the personnel responsible for the reliable operation of the nickel bath. Note that this is not very difficult, since the bath gradually becomes alkalized, and, therefore, there is a need to add pure sulfuric acid. Prepare a solution containing 25% (vol/vol) concentrated acid and 75% (vol/vol) distilled water, and add it in small portions to the bath with constant stirring. To control pH, it is enough to have a limited set of indicator paper, for example, three to five ranges produced by PNR.
The color scale on this paper is not as expressive as on Merck papers, but after a certain time the operating personnel will gain experience and will be able to read pH values with sufficient accuracy. Failure to comply with the required pH value will result in a noticeable deterioration in the quality of coatings. At a very high pH value, i.e. with insufficient acidity, coatings become brittle and prone to peeling, and also acquire a yellow tint; Burning of the coating also easily occurs in places of high current densities. At a pH less than 4, the gloss of the coating is weakened. A nickel bath is easily contaminated with metal impurities, especially when processing brass and zinc products: most often - copper, zinc, iron and lead. Copper gives nickel plating its dark color.
A low concentration of zinc leads to the appearance of black dots on the nickel coating, a high concentration of zinc manifests itself in the form of blackening of the coating in places of low current densities; Heavy zinc contamination can cause black streaks to spread over the entire surface. Iron contamination leads to an increase in internal stresses in the coating, resulting in the brittleness of nickel. A colloidal suspension of iron compounds appearing in a nickel bath can cause severe pitting.
Lead contamination appears as a brown or silver-brown layer in areas of low current density. Lead can enter the bath from lead pipes used for heating or from immersion heaters placed in a lead casing. This is extremely harmful for baths with shiners. Impurity metals are removed electrolytically at low current density using the method described when preparing the bath. Cleaning time depends on the degree of contamination of the bath and can last from several hours to several dozen. After a certain time, the steel sheets should be removed from the bath, cleaned with a steel brush and placed back into the bath. You should not leave de-energized sheets in the bath, as this leads to at least partial dissolution of contaminants in it.
Modern nickel plating baths are mixed with air and, therefore, mechanical impurities settling at the bottom are distributed throughout the entire volume of the bath and some of them enter the coating, giving it roughness. Thus, there is a need for continuous filtration of the bath, although most workshops avoid this, limiting themselves to periodic filtration, and do not complain about the roughness of the coatings. This indicates the ability to maintain the bath in proper cleanliness, first of all, the impossibility of getting anode sludge into the bath due to its good retention by the anode bag.
There are different opinions regarding the filtration of bright nickel plating baths using activated carbon. Theoretically, during each bath filtration, carbon should be used to remove harmful organic contaminants, which include, among other things, decomposition products of shine-forming additives. In fact, coal also absorbs some organic additives necessary for normal operation, as a result of which the consumption of rather expensive drugs, for example, “DF-bis” increases.
Despite this unfavorable situation for the consumer, it is necessary to choose a middle ground here, which is that filtration through fresh activated carbon occurs once a week and, finally, the frequency of carbon replacement can be set based on your own observations. Complete abandonment of coal is a technical mistake, since over time the bath becomes so contaminated with organic compounds that the coatings become brittle and passivated, making their decorative chrome plating difficult.
However, it is necessary to pay attention to the type of activated carbon. In Poland, Carbonol S-extga coal is used in dusty form. Random varieties can do more harm than good as they are contaminated with impurity metals such as zinc.
Despite great care of the nickel bath, it can become so contaminated with organic substances that a major regeneration using potassium permanganate becomes necessary. The contents of the bath, heated to 60 °C, are pumped into a spare bath and the pH is adjusted to 5.5-5.8 using nickel carbonate. Permanganate dissolved in water is added in small portions until a pale pink color is obtained. Thorough mixing is required during the administration of permanganate.
For each liter of bath thus treated, add 3 g of activated carbon and stir vigorously for several hours. Then leave the bath for 10-12 hours, after which the clear solution is carefully filtered into the working bath, without touching the sediment at the bottom. If the pink color remains, then add perhydrol, diluted in distilled water in a ratio of 1:5 until the normal green color of the bath is obtained. To prevent excess perhydrol, it is added in small portions. After adjusting the pH, test products are nickel-plated, remembering that the shine will be worse, since part of the shine-forming solution has been destroyed and only after adding shine-forming agents will the desired decorative appearance be obtained.
Iron, which contaminates the nickel bath, is removed mainly during the treatment described above at low current density, however, to more completely remove this metal, proceed as follows: the electrolyte is poured into a spare bath, heated to a temperature of 60 C and alkalized to pH = 6 nickel carbonate or caustic soda, and stirring is necessary. Then add perhydrol in an amount of 1 cm3/l, stir for 3 hours and leave until the next day. The light solution is carefully filtered into the working bath, making sure that the sediment at the bottom of the bath does not fall into the clean bath.
After bringing the pH to normal, test nickel plating is carried out and glitter-forming additives are added until the desired gloss is obtained. The correct dosage of brightening agents is the main requirement for success in bright nickel plating. Manufacturers of brighteners give approximate consumption of the substance depending on the amount of electricity passed in ampere hours, but few workshops have electric meters, and recording the load of the bath in the form of detailed records is not as simple as it seems.
During long-term operation of the bath, various situations arise, which are accompanied by a significant loss of brightening agents, for example, during the regeneration of the baths described above. This happens outside of electricity.
The content of the brightener in the bath can be determined analytically, but for this you need to have the appropriate equipment and a thorough knowledge of laboratory techniques. You can use the services of special laboratories, but this is not always possible. Consequently, it remains the own initiative of the galvanizer servicing the bath, or another more competent person. If we get a semi-shiny coating, this means that there is very little shine-forming agent in the bath. For 100 liters of bath, you can add 25 cm3 of the “DF-bis” additive. If this does not lead to an improvement in shine, then the reason lies in the bath itself. The pH and temperature of the bath should be determined and, if after adjusting these parameters there is still no improvement, then it is necessary to begin cleaning the bath using activated carbon and electrolysis at low current density, which is described in detail when considering baths.
A very difficult situation arises in the case of excessive introduction of brightening agents. In this case, the coatings become brittle in areas of very low current density; for example, in places where the product comes into contact with the pendants, visible black spots form and there may even be final defects in the coatings. Therefore, it is necessary to get rid of excess brightening agent by working the bath under normal conditions, hanging waste steel sheet on the cathode rod, degreasing them accordingly and etching them.
One of the common defects of nickel coatings is porosity - the so-called pitting - small depressions in the coating, reminiscent of pinholes, that occur during nickel plating as a result of the adhesion of hydrogen bubbles to the cathode surface. This is due to the high surface tension, especially in nickel plating baths. A coating does not form at the location of the bubble; a crater appears.
In addition to hydrogen, air bubbles contained in the bath can also settle on the surface of the cathode. The idle bath cools and absorbs a certain amount of air. When the bath is heated, air is released in the form of bubbles, some of which settle on the surface of the cathode, leading to pitting. Before starting nickel plating, the bath should be heated to a temperature several degrees higher than usually used, turn off the heat and wait until the temperature drops to normal. In addition, air may enter the bath due to a leak in the filter pump. Pump leaks should definitely be eliminated.
The condition of the substrate also has a great influence on the formation of pitting, the porosity of which, non-metallic inclusions and all kinds of surface contamination contribute to the formation of pitting. Contamination of the bathtub with suspension or decay products of organic substances also affects the bathtub in the same way.
To prevent the formation of pitting, oxidizing or wetting agents are added to the bath. The first includes hydrogen peroxide, added to ordinary baths in the form of perhydrol in quantities of 0.2-0.5 cm3/l.
Perhydrol is not added to baths with shine formers, as it has a destructive effect on organic substances. In such cases, wetting agents, for example, sodium lauryl sulfate, are added in an amount of 0.1-0.2 g/l.
Hydrogen peroxide and wetting agent are not radical anti-pitting agents. Mechanical shaking of products on suspensions in the bath helps eliminate gas bubbles. A movable cathode rod, or even better, mixing the bath with compressed air, makes the fight against pitting much easier. For persistent pitting, bath cleaning with activated carbon should be used.
Influence of electrolysis mode on coating quality and current efficiency.
The properties of nickel coatings are greatly influenced by:
- Bath composition;
- Temperature;
- pH;
- Current density;
- Foreign impurities.
5.1 Influence of electrolyte composition on the properties of nickel coatings.
All nickel plating electrolytes are divided into the following main groups:
- Sulfate (Watts);
- Sulfamine;
- Fluoroborate;
- Chloride;
- Fluorosilicic.
In practice, the first two are most often used. The remaining electrolytes are intended for producing matte nickel at high current densities, with 100% current output, or for coating aluminum products directly by electrolysis. All of them are much less common.
Watts' electrolyte can be used to produce both matte and shiny coatings. Currently, about 80% of all nickel coatings are bright. It is also possible to obtain shiny coatings from sulfamine electrolyte with certain additives, but more often it is used to obtain matte ductile nickel with low internal stresses for electroplating or metallization of dielectrics.
The introduction of shine-forming and leveling additives into the electrolyte allows you to obtain smooth and shiny coatings straight from the bath. Bright nickel plating has a number of advantages over matte nickel:
- the labor-intensive operation of mechanical polishing is eliminated;
- metal consumption is reduced due to mechanical polishing on corners, edges and edges;
- the number of technological operations is reduced and conditions are created for automation of the entire technological cycle;
- the deposition process is intensified due to the use of higher current densities.
The main disadvantages of shiny coatings compared to matte ones are strong hydrogenation, the presence of increased internal stresses and a large number of impurities that impair mechanical properties.
The current efficiency in a sulfate electrolyte increases with increasing concentration of nickel ions in the electrolyte.
The effect of chloride concentration on the physical and mechanical properties of nickel coatings is shown in Figure 5.
Figure 5 - Effect of chloride concentration on elongation, internal stress, hardness and tensile strength of electrodeposited nickel from Watts electrolyte solutions at pH=3.0, temperature 55°C and current density 5A/dm2.
Let us take a closer look at the effect of additives in the nickel plating electrolyte. All additives to the nickel plating electrolyte are divided into shine-forming, anti-pitting (wetting), leveling, and electrically conductive. They can be of both organic and inorganic origin. Many additives are not used today as they are obsolete (phthalimide, formalin, chloramine B, etc.).
5.1.1 Brightening agents in nickel plating electrolyte.
According to one of the accepted classifications, shine-forming additives are divided into two classes:
- Weak shine formers (class I)
make it possible to obtain shiny coatings only on a polished surface; their shine is inversely proportional to thickness. They do not affect cathodic polarization. These include methenamine, saccharin, chloramine B, disodium salt of naphthalene-1,5-disulfonic acid and others. - Strong shine formers (II class)
help to obtain shine not only on polished, but also on matte surfaces, and the shine does not depend on the thickness of the coatings. They increase cathodic polarization and level out the microrelief, but worsen the mechanical properties of deposits. These include coumarin, thiourea, 1,4-butynediol and others.
It has been established that brightening agents of the second class, especially those with double and triple bonds, are, as a rule, hydrogenated during electrolysis, and the sulfo groups of brightening agents of the first class are ultimately reduced to sulfide. Nickel sulfide, when included in the sediment, deactivates the catalytic centers of nickel, slows down the parallel reaction of the discharge of hydrogen ions and the hydrogenation processes of brighteners, and reduces the hydrogenation of sediments. This explains the advantageous use of both strong and weak shine formers.
Currently, among the brighteners, butynediol-saccharin binders are most often used due to the fact that their behavior has been best studied, and methods for purifying the electrolyte from the decomposition products of these additives have been developed.
5.1.2 Leveling additives.
Leveling additives, being surfactants, block protruding parts of the surface, and therefore deposition occurs in microcavities. Leveling additives include, for example, NIB-3. Some strong shiners also have a leveling effect.
5.1.3 Wetting agents.
Nickel plating is characterized by a phenomenon called pitting. Bubbles of hydrogen gas are retained on the cathode surface and in these places further discharge of nickel becomes impossible. Nickel begins to discharge near the bubbles (Figure 6). Pores appear on the coating, and it loses its protective and decorative properties. The adhesion of bubbles to the cathode is facilitated by all substances that increase surface tension. Hydroxides and organic compounds, as well as their decomposition products and even dust, can have a strong impact.
Figure 6 - Scheme of pitting formation on a nickel coating.
Wetting additives help reduce the surface voltage of the electrolyte and remove dirt and hydrogen bubbles from the cathode surface. Wetting additives include isoprylnaphthalene sulfonic acid, sodium lauryl sulfate, Progress detergent, etc.
It should be noted that most wetting and shine-forming additives are sulfo compounds. During electrodeposition, nickel sulfamide is formed as a result of a series of transformations. The sulfur content in sediments in large quantities adversely affects the mechanical and corrosion properties of the latter.
5.1.4 Electrically conductive additives.
Mainly to increase the electrical conductivity of the solution and, accordingly, reduce the voltage on the bath and save electricity, inorganic salts are introduced into it. Most often it is sodium, potassium or magnesium sulfate. However, as will be said in paragraph 2.6, these additives contribute to alkalization of the near-cathode layer and deterioration of the quality of the coating.
5.2 Influence of impurities in the electrolyte on the quality of nickel coating.
Nickel deposits are very sensitive to impurities entering the electrolyte. These impurities are divided into inorganic and organic.
Inorganic impurities cause the formation of brittle, cracking deposits. In the presence of copper, the deposits become rough. Zinc impurities cause dark and even black streaks to appear. In the presence of even small impurities of lead, a dark, scaly coating is formed that easily crumbles. Of the anions, the most harmful is NO3-, which promotes peeling of coatings. Such electrolytes are unsuitable for use. Chromic acids have a similar effect.
Organic compounds cause pitting, brittleness and roughness of coatings. If the electrolyte is contaminated with organic matter, it is impossible to obtain shiny precipitates.
5.3 The influence of temperature and stirring of the nickel plating electrolyte on the quality of coatings.
As the temperature increases, the current efficiency of nickel increases, since due to the acceleration of the diffusion process, polarization decreases - the nickel deposition potential becomes more positive. In this case, the hydrogen overvoltage changes slightly. Stirring the solution has the same effect.
The effect of electrolysis temperature on the physical and mechanical properties of nickel coatings is shown in Figure 7.
Figure 7 - Dependence of elongation, tensile strength and hardness of nickel coatings on electrolyte temperature at pH = 3.0 and current density 5 A/dm2.
5.4 Influence of electrolyte pH on the quality of coatings.
Nickel ions in the electrolyte are surrounded by a shell of dipole water molecules. In the electric double layer, some of the water molecules come off. Dehydration of the last water molecules requires energy, which is manifested by an increase in overvoltage, called chemical polarization. In this case, the equilibrium potential of nickel even at low current densities becomes negative.
At low pH values <1-2, nickel is almost not deposited and only hydrogen is released at the cathode. As the pH increases, the potential for hydrogen evolution becomes more negative and conditions are created at the cathode for the joint evolution of hydrogen and nickel. In this case, the higher the pH, the lower the proportion of hydrogen evolution. At high pH values, nickel precipitation cannot be carried out, since hydrolysis begins. Hydrolysis products (nickel oxide and hydroxide), penetrating into the coating, help retain hydrogen bubbles on the cathode surface, so the deposited nickel becomes porous, rough and dark. At very high pH values, a green precipitate of insoluble nickel salts can be seen on the parts with the naked eye.
Solid, stressed sediments are formed at pH >5.5, especially at temperatures below 20 °C. An increase in temperature leads to a slight decrease in internal stresses. Sediments obtained at low pH values are softer and more elastic.
The effect of pH on the physical and mechanical properties of nickel coatings is shown in Figure 8.
Figure 8 - Effect of pH on internal stress, tensile strength, ductility and hardness of nickel electrodeposited from Watts electrolyte at a temperature of 55° C and a current density of 5A/dm2.
5.5 Influence of current density and its shape on the quality of coatings.
Increasing the current density within specified limits helps reduce internal stresses and increase the gloss of coatings. The higher the temperature, the wider the operating range of current densities. It should be remembered that when the current density is significantly increased, the coating changes from fine-crystalline (compact) to dendritic (powdery) due to a violation of the stability of the flat growth front of the deposit.
The effect of current density on the physical and mechanical properties of nickel coatings is shown in Figure 9.
Figure 9 - Effect of current density on internal stress and hardness of nickel electrodeposited from Watts electrolyte at pH=3.0 and temperature 55° C.
Promising today is the use of non-stationary modes of electrolysis and ultrasound. Nickel plating with current reversal and ultrasound exposure is carried out in sulfate electrolytes. Nickel can be passivated by anodic polarization, so deposition is carried out with short anodic pulses or intermittent current. This results in the activation of nickel during the plating process. Reversing and interrupting the current ensures the production of coatings with low porosity, low internal stresses and high protective properties.
The sequential use of asymmetrical and direct current has a positive effect. Direct current deposition can improve the shine and hardness of nickel coatings.
Electrodeposition of nickel by pulsed current makes it possible to obtain mirror-shiny coatings from conventional nickel plating electrolytes without the use of shine-forming additives.
The application of ultrasound, as well as the combined use of ultrasound and reverse current, can significantly intensify the electrodeposition process. At the same time, the permissible deposition current density increases, electrolytes of conventional composition produce light, durable and practically pore-free deposits with very small coating thicknesses, at the same time the gloss of the coatings improves, and internal stresses are reduced.
To use non-stationary electrolysis modes, it is necessary to use rectifiers with special functions.
5.6 Comparison of physical and mechanical properties of nickel coating layers obtained under different conditions from Watts electrolyte and sulfamate solution.
Figure 10 - Schematic illustration of the influence of the above-described parameters of nickel deposition from Watts electrolyte and sulfamate solution on the physical and mechanical properties of the coating.
Nickel anodes
Nickel anodes are manufactured in accordance with the PN-82/N-92914 standard with dimensions of 25x25x(3-8) mm and sheets 100-300 mm wide, 600-1000 mm long and 6-12 mm thick. The chemical composition of the anodes must comply with the PN-79/N-8181 standard, and the sheets - with the PN-79/82180 standard. Anodes in the form of sheets have grades No. 2, No. 3 and No. 6A. For bright nickel plating, anodes No. 2 are used. An example of anode designation when placing an order: nickel anode No. 2 8Х300Х600 in accordance with standard RN-82/N-92914. The cubes are placed in titanium baskets. Removing Nickel Plating Nickel coatings on steel and copper alloys are typically removed in a dilute sulfuric acid bath. To 20 liters of cold water, add 30 liters of concentrated sulfuric acid in portions with constant stirring, making sure that the temperature does not exceed 60 ° C. After cooling the bath to room temperature, its density should be ~1.63. In order to reduce the risk of seeding of the substrate material, 50 g/l glycerol is added to the bath. Bathtubs are made of vinyl plastic. The products are hung on the middle rod connected to the positive pole of the current source. The side rods, on which lead sheets are hung, are connected to the negative pole of the current source. The bath temperature should not exceed 30 °C, since the hot solution acts aggressively on the substrate. The current density reaches 4 A/dm2, but the voltage can be varied within 5-6 V. After a certain time, concentrated sulfuric acid should be added to maintain a density of 1.63. In order to prevent dilution of the bath, it is necessary to immerse the products in the bath after pre-drying them. In the case of brass products, controlling the process is not difficult, since at the moment of complete removal of nickel, the current density drops sharply.
Black Nickel Platings
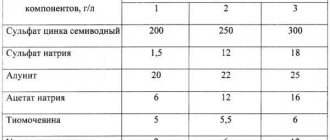
Black nickel coatings are used for decorative and special purposes. Their protective properties are very low, so they are applied to an underlayer of zinc, cadmium or ordinary nickel. Steel products are pre-galvanized, and brass and copper are nickel-plated. Black nickel plating is hard and brittle, especially when thick. In practice, a thickness of 2 microns is usually satisfied. The bath for applying such coatings contains a significant amount of zinc and thiocyanate. The coating contains ~50% nickel, with the rest being zinc, sulfur, nitrogen and carbon. Below are typical compositions of black nickel plating baths, g/l:
| Bath | 1 | 2 | 3 |
| NiSO4•7H2O | 75 | — | 144 |
| NiSo4•(NH4)2SO4•6H2O | 45 | — | — |
| ZnSO4•7H2O | 38 | — | — |
| NaSCN•2H2O | 15 | 15 | — |
| NiCl2•6H2O | — | 75 | — |
| NH4Cl | — | 30 | — |
| ZnCl2 | — | 30 | — |
| (NH4)6•Mo7O24•4H2O | — | — | 30 |
| H3BO3 | — | — | 23 |
Bath 1 operates at room or slightly elevated temperature, J = 0.1–0.5 A/dm2, pH = 5.6–5.9.
Bath 2 is chloride and, therefore, more aggressive than sulfate. It operates at room temperature, pH = 5.0 and J = 0.2 A/dm2. Bath 3 contains molybdates and is therefore more expensive than the first and second baths. Its advantage is high covering power and stability, as it contains boric acid. The disadvantage is the higher operating temperature, usually above 50 °C. The current density is 0.2–0.5 A/dm2, pH = 4.3–4.7. Black nickel plating baths are prepared by dissolving all components in warm water and filtering through filter paper. If difficulties arise in dissolving boric acid in bath 3, then it is dissolved separately in water heated to 70 °C. The stability of the baths lies mainly in the control and regulation of pH using H2SO4 or NaOH. Achieving deep black color depends mainly on the correct choice of current density. Nickel-plated products are lubricated with hot oil.
Metal nickel plating technology
Nickel plating is carried out by applying a thin layer of nickel coating to a metal object. Products made from various metals can be plated with nickel, such as:
- steel;
- copper;
- titanium;
- aluminum.
There are metals that cannot be nickel-plated:
- tin;
- lead;
- cadmium;
- antimony.
Nickel coating protects the product from moisture and various aggressive substances. It is often applied as a base layer before chrome plating parts. After applying a thin film of nickel, platings of silver, gold and other metals are held more firmly.
At home, methods are used that do not require the use of specialized equipment. Thanks to this, nickel plating of steel, copper, and aluminum at home is accessible to almost every person. To obtain a uniform coating, you must first prepare the part.
Advantage of nickel plating
Electroless nickel plating
This process was at one time one of the main ones in the technical literature, and it seemed that it created strong competition for traditional electrolytic nickel plating. Currently, it is assessed more calmly and used when there is technical and material support for this.
The main advantage of electrochemically applied nickel coatings is the uniform thickness regardless of the shape of the product. This is typical for all metal deposition processes without the use of current.
A feature of chemical nickel plating is the continuous deposition of a layer, which creates the possibility of forming coatings of any thickness.
Baths for chemical nickel plating consist of nickel salt, sodium hypophosphite and additives. The basis is nickel salts and sodium hypophosphite.
There are two types of baths for chemical nickel plating - acidic and alkaline. Nickel sulfate or chloride of relatively low (~5 g/l) concentration is mainly used as nickel salts. The content of hypophosphite reaches 10-30 g/l. Additives are introduced in the form of complexing compounds that accelerate the deposition of nickel, and stabilizers that prevent the decomposition of the electrolyte.
Glycolic, lactic, citric and aminoacetic acids are mentioned as complexing compounds in the specialized literature. Accelerators are succinic, malonic, propionic, butyric, valeric and other acids. Lead compounds, thiosulfate, thiourea, etc. are mainly used for stabilization.
Below are examples of two baths for electroless nickel plating, g/l:
| Bath | 1 | 2 |
| Nickel sulfate (NiS04•7H2O) | 20—30 | — |
| Sodium acetate (CH3•COONa•3H2O) | 10—15 | — |
| Lactic acid (CH3CHOHCOOH) | 25—30 | — |
| Thiourea (H2NCSNH2) | 0,0005—0,001 | — |
| Sodium hypophosphite (Na2HPO2•H2O) | 15—20 | 15—25 |
| Ammonium chloride (NH4Cl) | — | 30—40 |
| Sodium citrate (Na3C6H5O7•5.5H20) | 30—50 | |
| Ammonia (NH4OH) | 70-100 | |
| Nickel chloride (NiCl2•6H2O) | 20—30 |
Bath 1 is acidic, works best at pH = 4.3-4.8.
The operating bath temperature of 85–90 °C must be maintained during the entire nickel plating process. To regulate the pH, use a diluted (for example, 5%) solution of sodium hydroxide. Prepare bath 1 as follows: in distilled water heated to a temperature of 60 ° C, first dissolve sodium acetate, then nickel sulfate and add lactic acid, previously neutralized with sodium hydroxide to pH = 3.5-4.0. After heating the bath to 85 °C, add sodium hypophosphite. After this, you can begin nickel plating.
The concentration of thiourea is very small and in a workshop there is no possibility of weighing with an accuracy of fractions of a gram. Since excess thiourea can completely delay the nickel plating process, it is better to avoid this stabilizer completely and use a thiourea-free bath. Bath 2 is alkaline. Sodium citrate, ammonium and nickel chlorides are dissolved in distilled water heated to 60 °C, and an ammonia solution is added in portions with constant stirring to achieve pH = 8-9. In this case, a noticeable change in the color of the solution occurs from greenish to blue. After heating to 80 °C, hypophosphite is added and the bath is ready for use.
The information provided is very general and does not reflect much of the practical side of nickel plating. Below 80°C the bath efficiency is very low. At 90 °C, a nickel layer 10–20 μm thick is obtained within 1 hour. With a further increase in temperature, for example to 95°C, thicker layers are obtained, but the stability of the bath decreases. At a certain point, sudden decomposition of the bath may occur, which is accompanied by the appearance of black powder on the bottom and walls of the bath. This bath is unsuitable for further use.
A major problem is the selection of appropriate working containers. In industrial settings, complex installations made of corrosion-resistant steel are used, while for small-scale nickel plating, glass, porcelain or enamel containers are mainly used. The best way to heat small and medium-sized containers is with a water jacket. Let's lower a 5 liter glass vessel into an enameled 10 liter tank of water, we can get a water jacket suitable for gas or electric heating to boiling point. In a glass vessel, you can reach a temperature of 83-85 ° C, sufficient to carry out the process.
High temperature and strong gas evolution on the surface of the products are detected by the service personnel by a strong unpleasant odor. Obviously, the entire installation must be under a hood. As you can see, the whole procedure is not simple, as a result of which the use of chemical nickel plating is limited to those cases where electrolytic methods are not applicable. For example, metal bellows in the form of a cylindrical accordion, used to measure pressure during pneumatic adjustment, cannot be electrolytically plated with nickel due to their complex geometric shape. Electroless nickel plating is an excellent solution to this problem.
Steel products can be chemically nickel-plated without difficulty. On copper and brass, nickel deposition begins after brief contact with a less noble metal, such as iron or aluminum. For nickel plating of aluminum alloys, alkaline baths are usually used (for example, bath 2). Small particles of nickel may settle on the walls and bottom of a vessel used for chemical nickel plating, especially if the surface of the vessel is not very smooth or has scratches. Before further use of such a vessel, it is necessary to remove deposited nickel particles by dissolving them in nitric acid.