Calcium carbide is a compound of calcium with carbon, which is a solid crystalline substance. It is produced by fusing calcium oxide with coke in electric furnaces (at temperatures of 1900-1950°C) and subsequent solidification in special forms (molds), crushing and sorting into pieces of certain sizes (small, medium and large).
Thus, a technical product with a dirty dark gray or brown color is formed due to the 20-25% content of impurities (coal and other coloring matter). In addition, it contains calcium sulfide and phosphide, due to which the material has an unpleasant odor.
It absorbs water well and when interacting with it, even at low temperatures, it decomposes and violently releases acetylene gas with a large amount of heat. Even atmospheric moisture can provoke decomposition of the substance.
Physical properties
- Colorless tetragonal crystals.
- Density: 228 (+20 °C, g/cm3).
- Specific heat capacity at constant pressure (in J/g·K): 0.92 (+20–325 °C).
- Standard enthalpy of formation ΔfH (298 K, kJ/mol): −62.8 (t).
- Standard Gibbs energy of formation ΔfG (298 K, kJ/mol): −67.8 (t).
- Standard entropy of formation S (298 K, J/mol·K): 70.3 (t).
- Standard molar heat capacity Cp (298 K, J/mol·K): 62.34 (t).
- Melting enthalpy ΔHmelt (kJ/mol): 32.2.
- Calcium carbide has a strong garlicky odor.
Production[edit]
Calcium carbide is produced industrially in an electric arc furnace from a mixture of lime and coke at a temperature of about 2,200 °C (3,990 °F). [6] This is an endothermic reaction requiring 110 kilocalories (460 kJ) per mole [7] and high temperatures to remove carbon monoxide. This method has not changed since its invention in 1892:
CaO + 3 C → CaC 2 + CO
The high temperature required for this reaction is practically unattainable with traditional combustion, so the reaction is carried out in an electric arc furnace with graphite electrodes. The resulting carbide product typically contains about 80% calcium carbide by weight. The carbide is crushed to form small lumps ranging in size from a few to 50 mm. Impurities are concentrated in smaller fractions. The CaC 2 content of the product is determined by measuring the amount of acetylene formed during hydrolysis. For example, British and German standards for larger fractions are 295 L/kg and 300 L/kg respectively (at 101 kPa pressure and 20 °C (68 °F)). Impurities present in carbide include phosphide, which produces phosphine upon hydrolysis. [8]
This reaction was an important part of the Industrial Revolution in chemistry and was made possible in the United States as a result of the enormous amount of inexpensive hydroelectric power produced at Niagara Falls until the early 20th century. [9]
The production method in an electric arc furnace was discovered in 1892 by T.L. Wilson and independently H. Moissan in the same year. [10] [11] [12] In Bosnia and Herzegovina, in the city of Jajce, the Austrian industrialist Dr. Joseph Kranz and his
, which was later succeeded by
Elektro-Bosna
, opened the largest chemical plant for the production of calcium carbide at that time in Europe in 1899. The hydroelectric power plant at Plivedla power supply plant was built on the river with an installed capacity of 8 MW. It was the first power station of its kind in South-Eastern Europe and was commissioned on 24 March 1899 [13].
Appearance and characteristics of technical calcium carbide
Calcium carbide is produced by fusing coke and quicklime in electric furnaces. Molten calcium carbide is released from the furnace into special forms - molds, in which it hardens. The solidified calcium carbide is crushed and sorted into pieces of certain sizes.
Technical calcium carbide is a solid crystalline substance. In appearance, calcium carbide is a dark gray or brown solid. It produces a gray crystalline fracture with varying shades depending on purity. Calcium carbide greedily absorbs water. When interacting with water, even in the cold, calcium carbide decomposes with the rapid release of acetylene and a large amount of heat. The decomposition of calcium carbide also occurs under the influence of atmospheric moisture.
According to GOST 1460-56, the following dimensions (granulation) of calcium carbide pieces are established: 2×8; 8x15; 15×25; 25x80. Technical calcium carbide contains up to 80% of chemically pure calcium carbide, the rest is made up of impurities - quicklime, carbon, silicic acid and more.
Welding applications
Carbide is stored in special steel tanks with a volume of 100 or 130 liters. These tanks should only be opened when there is no fire or sparks in the vicinity using a wooden hammer and a brass chisel. Unused carbide in the jar is sealed with a waterproof lid.
Acetylene for welding is produced from carbide in a generator of stationary or mobile type and of different volumes. The average volume of acetylene generators is designed to receive from 5 to 15 liters of water and, accordingly, 2-5 kilograms of carbide. The yield of acetylene is considered slightly lower than theoretical and is taken to be 260-280 liters per kilogram of CaC2. It is recommended to use coarse carbide – 80 mm
The principle of using acetylene for welding work is as follows:
- About 250 liters of acetylene are released from one kilogram of carbide, and 3-4 liters of water are required to decompose a kilogram of carbide. Knowing these proportions, the required volume of water and the amount of substance are calculated.
- In industrial generators, designed for a long and uniform process of use, carbide is dosed into the gas-forming chamber through a special hopper in automatic mode. In generators that are used for non-standardized volumes of work, the carbide is immersed in water in a special basket. The volume of acetylene produced is regulated by immersing or raising the basket.
- When the next portion of carbide is supplied and the reaction begins, the pressure in the chamber increases, which is reduced by the active release of acetylene into the burner.
- Acetylene is fed through a hose into the gas burner. The burner must be located at least 10 meters from the generator.
- The slaked lime formed during the reaction (about 1.2 kg per kilogram of carbide) is removed from the generator through a separate hopper.
In gas welding, the main advantage of using carbide is its low weight and the light weight of the equipment used. Gas cylinders for acetylene are very heavy; they must be moved on a special trolley or with 2-3 pairs of hands. The average generator weighs 15-20 kg, which allows you to move it without much effort alone or with one assistant. When moving dry carbide, it is enough to follow basic storage rules - avoid getting moisture on the substance and getting fine carbide dust on your skin and eyes.
Transportation and storage
Calcium carbide powder decomposes almost instantly when exposed to moisture. This produces acetylene, which in high concentrations is flammable and explosive. That is why it is necessary to pay quite a lot of attention to the storage of calcium carbide, for which cans and special drums are often used. Other storage features include the following:
- The released acetylene is lighter than air, so it accumulates at the top. It is worth considering that it has narcotic effects and can spontaneously ignite.
- When producing large volumes of a substance, special attention is paid to safety precautions. Special packaging is used for packaging.
- To open the package, use tools that do not cause sparks.
- If the substance gets on the skin or mucous membrane, it must be removed immediately. In this case, the affected surface is treated with a special cream or other protective and healing substance.
- According to established rules, transportation can only be carried out using a covered vehicle. However, delivery by air is prohibited.
Transport container
Regulations also prohibit storing calcium carbide with other chemicals and heat sources. This is because the resulting gases can react chemically with other chemicals and ignite.
In reducing CO2 emissions
The residues obtained from the production of acetylene from CaC2 (also called "calcium carbide residue" or "calcium carbide residues") are used to produce clinker or concrete.
Calcium carbide mud has a high content of calcium hydroxide (Ca(OH)2) (about 90%), some calcium carbonate (CaCO3) and has a pH greater than 12.
For these reasons, it can react with SiO.2 or Al2OR3 to form a product similar to that obtained during cement hydration.
One of the human activities that produces the most CO2 emissions is the construction industry. Cooperation2 it is formed in the east to separate from calcium carbonate during the reaction to form concrete.
Using calcium carbide sludge to replace calcium carbonate (CaCO3) has been found to reduce CO2 emissions by 39%.
Links[edit]
- https://materialsproject.org/materials/mp-1575/
- Massalimov, IA; Kireeva, M.S.; Sangalov, Yu. A. (2002). "Structure and properties of mechanically activated barium peroxide". Inorganic materials
.
38
(4): 363. DOI: 10.1023/A: 1015105922260. S2CID 91881752. - NFPA Hazard Rating Information for Common Chemicals. Northeastern University
- Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. ISBN 0-07-049439-8.
- ↑
Vincoli, Geoffrey Wayne (25 November 1996). Risk Management for Hazardous Chemicals. CRC Press. item 429. ISBN. 978-1-56670-200-3. - ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of elements
(2nd ed.). Butterworth-Heinemann. p. 298. ISBN 978-0-08-037941-8. - Calculated from data in the CRC Handbook of Chemistry and Physics
. - ^ ab Calcium Carbide, Bernhard Langhammer, Ullmann Encyclopedia of Industrial Chemistry, Wiley Interscience. (Subscription required)
- Freeman, Horace (1919). "Production of cyanamide". Chemical News and Journal of Physical Sciences
.
117
:232. - Morehead, J. T. and de Chalmot, G. (1896). "Calcium Carbide Production". Journal of the American Chemical Society
.
18
(4): 311–331. DOI: 10.1021/ja02090a001.CS1 maint: multiple names: authors list (link) - Moissan, H. (1892). "Chimie Minérale - Description d'un nouveau four électrique". Comptes rendus hebdomadaires des séances de l'Académie des Sciences
.
115
:1031. - Renouf, Edward (1899). "Uses of Acetylene". Monthly Popular Science Magazine
: 335–347. - "Zgrada Prve hidrocentrale na Balkanu - Komisija za očuvanje nacionalnih spomenika". old.kons.gov.ba
(in Serbo-Croatian). CONS. Retrieved March 15, 2022. - ^ab Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of elements
(2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. - ↑
Dong, I (23 January 2006). "Troubles in the PVC industry". Hong Kong Trade Development Council. Archived December 28, 2007.CS1 maint: bot: original URL status unknown (link) - "Government moves to curb development of calcium carbide sector". China Daily via BusyTrade.com. 2007-05-16. Archived February 11, 2007.CS1 maint: bot: original URL status unknown (link)
- Lacson, Jamie; Schlag, Stefan; Toki, Goro (December 2004). "Calcium Carbide". Research Institute Consulting.
- "Speleological equipment and culture (from the Te Ara Encyclopedia of New Zealand)".
- Clemmer, Gregg (1987). American Miners' Carbide Lamps: A Collectors' Guide to American Carbide Mines Lighting
. Westernlore Publications. - Abeles, F. B. & Gahagan, H. E. III (1968). "Uptake: the role of ethylene, ethylene analogues, carbon dioxide and oxygen". Plant Physiol. 43
(8):1255–1258. DOI: 10.1104/pp.43.8.1255. PMC 1087003. PMID 16656908. CS1 maint: multiple names: authors list (link) - “Bet on it. Your mango is ripened using carbide." DNA
. 2013-05-18. Retrieved August 25, 2022. - "Eating artificially ripened fruits is harmful".
- "Carbidschieten wordt feest" (in Dutch). Algemeen Dagblad. 2016-12-24.
- Singh, Randhir. "Determination of water content in soil - calcium carbide method". Civil Engineering Portal
. Retrieved September 7, 2022. - ASTM International. "ASTM D4944-18, Standard Test Method for Determining Water (Moisture) Content of Soil Under Field Conditions Using a Calcium Carbide Gas Pressure Meter". ASTM International. Retrieved September 7, 2022.
- du Bois, TME "Molehill Mayhem". paragraph 21.
- "How to Get Rid of Yard Moles with Carbide". mysunnylawn.com
.
What is carbide?
Homemade bombs. This is what comes to mind first when we hear the word carbide. And no, the production of these dangerous toys was not carried out by defense industry enterprises, but, as a rule, by boys, about ten years old.
Twenty years ago it was a favorite pastime among teenagers. Now everyone sits at their tablets, but back then the world was ruled by the inquisitive mind of a child who strove to try everything in practice.
In order to feel like Rimbaud, you needed to get one miracle stone. Children most often found them at construction sites. And then everything was simple: a plastic vessel, a stone, water, a tightly screwed cap. All this was zealously shaken, and at best, thrown away. And in the worst case, the “shell” exploded right in the hands, then injuries could not be avoided.
Calcium carbide
There were also safer ways to use the find, for example, simply throwing it into a puddle, then you could observe something similar to the effect of modern bath bombs. So what kind of popular “toy” is this? Most of us believed that nature produced carbide as we know it. But actually it is not. And today you will see this.
So, this substance is always very hard, plus, in order to melt it, you need to make a remarkable effort. They look like dark, light, greenish stones, or powder, it all depends on the composition. Its shelf life is short, usually six months. It will not be possible to place containers in a general warehouse; such potentially dangerous substances must have their own compartment.
As you already know, carbide constantly strives to explode. Moreover, some connections do not even need special conditions. It is enough just to pour the powder from container to container, and it can suddenly explode.
Properties and composition
To obtain this stone, you need at least two elements. First is carbon. Its presence is mandatory. And then there is a choice: metal or non-metal. The main thing is that the rule is followed: the electronegativity (the force with which the atoms of an element attract foreign electrons) of the required component is higher than that of its “partner”. Otherwise you will get completely different connections.
Calcium Carbide Formula
This connection was first discussed in England back in the 19th century. However, the fame of the discoverer went to the Frenchman, thanks to whose experiments the substance was officially recognized; this happened only towards the end of the century. And now about what qualities are inherent in this compound:
- The material is unusually hard. According to this indicator, it almost caught up with diamond. Among the record holders is tungsten carbide (9 out of 10 possible points). This opens up hundreds of ways to use it.
- It will take a lot of effort to melt the stone. After all, for this it is necessary to heat it to 2, or even 3 thousand degrees Celsius. This figure will be higher than the values necessary to change the state of metallic substances before they were included in the carbide.
- This is a very “non-contact” connection. Thus, the reaction of carbide to many substances will be zero. This requires special conditions. Therefore, they are not afraid of acids and other substances that promote corrosion.
- But the previous point does not apply to water. As you already understood from the story above, carbide and water often go hand in hand. In the case, for example, when calcium carbide is involved, absolutely any moisture is suitable for this, no conventions are needed. If the work is silicon carbide, then there is no way without heating - you need hot steam (1800 degrees).
Kinds
Science knows three types of such compounds:
- Covalent
What sets them apart is the very strong bonds between atoms. When this type is mentioned, we are talking about only two elements adjacent to carbon: the first is bromine, the second is silicon. All of the above properties in these compounds are “set” to the maximum. This is both unprecedented hardness and durability. If you want to dissolve it, it won’t be possible without the participation of caustic acids of enormous concentration. The same applies to interaction with oxygen. It just won’t work, you need heating, and not too bad – up to 1000 degrees.
- Salt-like or ionic
Here, either aluminum or metal comes into contact with carbon, but not just any metal, but only from 1-2 chemical groups. tables. It is still not very easy to give such a compound a liquid form; extreme heating is required. But the acid will not go unnoticed; as a result of such a “meeting,” the carbide will disintegrate.
- Metal-like
They are obtained from metals belonging to groups 4-8, this also includes cobalt, as well as nickel, and, of course, iron. If we consider their chemical structure, we will see that the carbon atoms are literally scattered, there are no bonds between them, they are like inclusions in the gaps formed in the metal. Because they are very refractory, one might even say they are champions in this matter. This allows them to be used in the manufacture of drills (pobedite drills).
Application
As already mentioned, this substance can most often be found at construction sites. And there they find dozens of ways to use it. It is difficult to do without this material in grinding; special materials are produced from it. disks. But it is good not only as an abrasive, but also in the form of sharp cutting wheels, knives and the like.
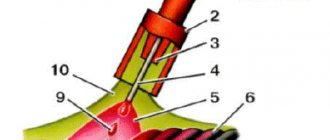
Acitylene generator for gas welding, inside of which calcium carbide is poured
Mechanical engineering is another opportunity to use this connection. Not only various car parts are made from carbide, but also spare parts. parts for radio devices. And thanks to its thermal conductivity, it will also cope well with heating tasks. Even in the nuclear industry it is impossible without such a component. All this requires special strength, so here we are most often talking about covalent types.
Those compositions that contain iron carbide make it possible to obtain steel, and the well-known cast iron. Silicon compounds are also valued by jewelers and manufacturers of lighting elements. Artificial rubber and resins, and even acetic acid - such is the wide range of applications of carbides.
But the matter does not stop there. This artificial mineral is also important for gardeners. After all, with its help a special type of fertilizer is obtained. They are able to regulate the growth rate of various crops.
But perhaps the most popular of all is calcium carbide. After all, it is precisely this that welders actively use in their work. It would seem, how can this dark pebble with a garlic aroma be involved in such a process?
It’s very simple, because gas welding, which is logical, requires flammable gas. In our case it gives acetylene carbide. As soon as it “meets” oxygen, we get a very intense flame; its temperature exceeds three thousand degrees.
If you take ready-made volatile gas, then special packaging is used for it. containers in which the substance is delivered to the site of action. There should be no shaking or shock during such a trip - it is deadly.
This raw material can flare up, even without extra “help”, so attention should always be at the limit. If the fire could not be avoided, do not use any moisture when extinguishing it. Only powder extinguishing methods should be used.
There is a second way - to produce this “fuel” right at the work site. To do this, you need to know what hydrolysis of carbides is. Simply put, it is the reaction of a compound upon contact with water. Moreover, even one drop can cause this very reaction.
Therefore, if you are planning to carry out welding work, very carefully open the sealed container with carbide. It is especially important that there are no signs of fire in the neighborhood, otherwise an emergency is guaranteed. You should completely forget about cigarettes.
Also, make sure that even the smallest crumbs do not end up on your skin, especially on the mucous membranes, otherwise, at best, there will be irritation, at worst, burns and swollen parts of the body. So arm yourself with special equipment. uniform: everything needs to be protected, from head to toe, including the respiratory tract. First aid, if contact cannot be avoided: pour plenty of water on the affected area, cover it with thick cream. If necessary, call a doctor.
If we talk about consumption, if the mass of carbide is one kilogram, then this makes it possible to produce up to three hundred cubic decimeters of gas. These are quite good indicators. Also, this amount of raw material will require approximately 20 liters of water, although manufacturers say that half a liter will be enough. How long this all takes depends on the size of the compound fractions and their purity.
After the work is finished, the remaining waste, and this is lime slag, is not left anywhere, but is disposed of. For such work you will need a specialist. generator. They come in impressive sizes and are installed in one place, for example, when large-scale work is planned. But there is also a mini version, portable.
First, fill the compartment in which the gas should form with water, then add carbide there. The reaction takes place, and the resulting acetylene flows through a soft tube directly to the gas burner. This path must be long enough; the hose must be chosen no shorter than ten meters.
Boron carbide
Boron carbide is also used. Items based on it provide reliable protection against fire. And not only from fire, by the way, because such products are actively used by manufacturers of body armor. Firstly, it “catches” bullets, and secondly, it will not allow radiation to pass through. As for such a union as aluminum carbide, the sparkling sparks during fireworks are its merit. But in appearance it is an unremarkable yellow powder.
How is carbide obtained?
First about calcium carbide. Its production is in demand. And although such factories require large expenses, especially when it comes to electricity, enterprises do not abandon the usual manufacturing method. Because the demand for such products is in no hurry to fall. After all, it is hardly possible to imagine at least a single construction site without acetylene. To save on electricity, such enterprises are opened in countries with a large number of hydroelectric power stations, in Canada, for example.
Why not switch to working with methane, since such a volatile gas can also be obtained from it? Yes, because calcium carbide produces an almost pure product, it is not difficult to bring it to 98 percent gas. And it is much easier to transport than the one obtained with the participation of methane.
The main object in such industries are electric furnaces. They are loaded with hard coal, which is also called coke, and calcium oxide (lime, any kind of lime will not work, it needs to be purified and homogeneous). All this heats up to 2 thousand degrees. And voila, the reaction started.
The result is a liquid substance, which will then become a familiar compound to us. But first it needs to cool in the molds. After the degree is reduced, these layers are crushed into pieces that are more convenient to use.
Now about the silicon version. We got it absolutely by accident, as usually happens. An American scientist tried to create an artificial diamond. As a result of the experiments, silicon carbides were obtained (by the way, they are in second place in hardness after uncut diamonds).
He patented it and opened the first plant for the production of the material. It’s impossible to say that technology has changed a lot since then. Unless sand and salt were excluded from it, what remained was carbon and silica, which are still heated to maximum temperatures in furnaces.
External links [edit]
- Calcium carbide and acetylene on the Periodic Table video
(University of Nottingham) - Calcium carbide production
- Material Safety Data Sheet for 2008
| vte Calcium compounds | |
| |
| Authoritative control |
|
Discontinued use
TsK2 it was used in so-called carbide lamps. They work by dripping water onto calcium carbide to form acetylene, which ignites and thus produces light.
These lamps were used in coal mines, but their use was discontinued due to the presence of CH4 methane in those mines. This gas is highly flammable and the flame of a carbide lamp may ignite or explode.
They were widely used in slate, copper and tin mines, and in early cars, motorcycles and bicycles as headlights or headlights.
Currently, they are being replaced by electric lamps or even LED lamps. However, they are still used in countries such as Bolivia, in the Potosi silver mines.