Operating principle and design of an electric arc metallizer
The principle of operation of the metallizer is to melt two wire electrodes with an electric arc and spray the molten metal with a jet of compressed air. Molten particles, falling on the surface to be coated, adhere to it and form a continuous coating, while the thickness of the layer is regulated by the number of passes of the metallizer and the speed of its movement relative to the metallized surface (Fig. 1).
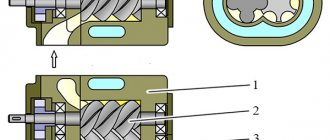
The design of the electric arc metallizer provides special guides through which two sprayed wires are continuously fed. An electric arc is excited between the ends of these wires. In the central part of the metallizer there is a nozzle through which compressed air is supplied. A jet of compressed air removes particles of molten metal from the electrode wires and carries them to the sprayed surface.
Rice. 1. Scheme of the electric arc metallization process : 1 – metallizer body; 2 – wire feed mechanism; 3 – air supply channel; 4 – electrode wires; 5 – electric arc with sprayed wire particles; 6 – sprayed coating
An electric arc metallizer can operate on both direct and alternating current. When using alternating current, the arc burns unsteadily and is accompanied by a lot of noise. With constant current, the nature of the work becomes stable, the sprayed material has a fine-grained structure, and the productivity of the process is quite high. Therefore, at present, direct current sources are used for electric arc metallization of surfaces. To operate the metallizer, wire with a diameter of 0.8...2 mm is usually used.
The advantage of the electric arc metallization method is the high productivity of the process and the possibility of significantly reducing the time spent on spraying. For example, with a current of up to 700 A, it is possible to spray a steel coating with a productivity of 30...35 kg/h, which is several times higher than the productivity of flame spraying. Compared to flame spraying, metallization allows one to obtain more durable coatings with a high degree of adhesion.
When wires made of two different metals are used as electrodes, it is possible to obtain a coating from their alloy. When spraying a coating by spraying two electrodes from dissimilar materials, it is desirable to use electrometallizers that would allow for separate adjustment of the feed speed of each electrode.
The disadvantage of the method under consideration is overheating and oxidation of the sprayed material at low feed rates of the sprayed wire. In addition, the large amount of heat released during arc combustion leads to significant burnout of the alloying elements included in the sprayed alloy. This must be kept in mind when developing coating technology and use wire containing a higher amount of alloying elements for spraying.
The metallizer usually consists of the following main parts: housing, wire feed mechanism drive, spray head, wire protective hoses and control panel. For example, the EDM-5U electric arc metallizer (Fig. 2) is designed for applying metal coatings with supersonic air flow onto a specially prepared surface. An asynchronous motor with a power of 0.25 kW allows you to work with wires with a diameter of up to 3.5 mm. The authors of this design (NPF TOM LLC) have developed a special supersonic air nozzle (air exit speed up to 500 m/s), which allows both to significantly increase the flight speed of molten metal particles (increase adhesion) and to reduce the spray angle, which leads to an increase in wire utilization factor. The metallizer control panel is equipped with a frequency converter for smooth regulation of spraying modes and connection of the metallizer with the welding power source.
In addition to the specified metallizer, metallizers of the EM-14M, EM-17, EM-19 type with various characteristics and performance have been developed and are used for applying corrosion-resistant coatings, restoring and strengthening the surfaces of products.
The power sources for the operation of metallizers are welding rectifiers such as VDU-504, -505, -506, VS-600, PSG-500, PSU-500 and others with adjustable voltage and a rigid current-voltage characteristic. These current sources make it possible to sputter almost any metal in a wide range of operating modes.
Rice. 2. Electric arc metallizer EDM-5U : 1 – electric motor; 2 – wire feed channels; 3 – nozzle; 4 – control unit; 5 – air supply channel
Marina Kapitsa
All articles in the series:
- Chemical metallization of dielectric. Part 1
- Chemical metallization of dielectric. Part 2
Creating a reliable electrical connection between the MPP layers and a conductive pattern on the dielectric surface is the most critical part of the MPP manufacturing process. Typically, this process consists of two stages: chemical metallization of the dielectric (formation of a layer of chemical copper) and growth of the copper layer by galvanic method to the required thickness. The chemical step is necessary to create an electrically conductive layer on the surface of the dielectric, onto which copper can be electroplated.
Coating technology
The technology for applying metal coatings consists of surface preparation, coating application and, if necessary, its processing.
Pre-treatment of the base surface is an important factor in ensuring strong adhesion of the coating to the part, since in most cases the connection of the sprayed coating to the base occurs as a result of mechanical adhesion. Therefore, in order for the molten particles that impact and deform on the substrate to firmly adhere to surface irregularities, the substrate must be sufficiently rough.
In addition to the mechanical connection of the sprayed coating with the base, other types of connections are possible, for example, the fusion of the sprayed material with the base material, the formation of chemical compounds, etc.
Increasing mechanical bond strength is associated with increasing the surface area of the substrate and creating greater substrate activity, which is also important for other types of joints. Therefore, creating a developed roughness on the surface of the base is an important requirement. Before pre-treating the surface, it is necessary to wash it and, as far as possible, remove moisture, oil and other contaminants, as well as oxide films.
In parts made of porous materials and cast iron castings, the pores may contain oil, which, when sprayed as a result of heating, is released onto the surface, which significantly impairs the adhesion of the coating to the base. Therefore, such parts, after normal degreasing, must be subjected to annealing at a temperature of 250...500 ° C, during which the oil contained in the pores burns out. Oxide films are removed from the surface mainly mechanically by blowing with quartz sand, corundum or steel chips. To remove oxide films from steel parts, etching in nitric, hydrochloric and other acids is sometimes used.
The usual means of preparing the surface of products with a complex configuration or rotating bodies is shot blasting with steel or cast iron crushed shot of granulation 0.8...1.6 mm, or corundum powder of the same granulation at an air pressure of 0.4...0.6 MPa, cleared of moisture and oils. For parts with an unhardened surface, surface preparation can be used by cutting ragged threads: for heat-treated hard surfaces, after thread cutting, blasting with electrocorundum powder is necessary. The values of the product surface roughness parameters, requirements for metallization, coating and control methods must comply with GOST 9.304–84 “Gas-thermal coatings. General requirements and control methods." The prepared surface should be metalized no later than two hours after completion of preparation.
The operating mode of the metallizer (voltage, metallization distance) is set by the operator depending on the substrate metal and wire used, its diameter, air pressure, and wire feed speed. When applying the coating, it is necessary to avoid heating the metallized surface above 100...120 °C.
To avoid overheating and peeling of the coating, it should be applied with continuous rotation of the part and reciprocating movement of the metallizer along its axis, or the metallizer should be moved relative to a stationary surface of a flat or complex configuration. High quality coatings can be achieved only if there is a continuous supply of wire and a minimum arc voltage, ensuring the stability of its combustion. Excessive voltage leads to overheating of the coating and excessive burnout of the alloying elements of the wire.
In an electric arc metallizer, the angle between the electrodes (sprayed wires) is usually 30...60°. At angles greater than 60°, the deposition process becomes sensitive to changes in deposition conditions and unstable. When the metallizer operates at direct current, the sprayed wire, which acts as an anode, melts approximately 50% faster than the cathode. Therefore, theoretically, the anode wire should be fed faster than the cathode wire. However, in practice there is no need for different electrode feed rates. Therefore, the wires are usually fed at the same speed, or the melting speed of the wires is controlled due to the difference in the diameters of the anode and cathode wires.
An important factor when spraying is the correct adjustment of the current, which allows you to balance the feed speed of the wires with the speed of their melting and thus ensure a constant arc length. When spraying, the distance from the metallizer nozzle to the surface to be coated is usually 100...200 mm (Fig. 3).
Rice. 3. Operation of the metallizer during coating
Chrome Plating Basics
Modern technology of chemical metallization allows the use of special paints and varnishes and reagents to develop spraying.
As a result, the coating will shine and reflect surrounding objects. In addition, it is chemical metallization that makes it possible to achieve the highest degree of adhesion. It is important that the coating process is carried out without the help of any caustic substances or explosive components. Carcinogenic components of chrome plating are reduced to an absolute minimum
Chemical metallization has no restrictions on the shape and size of the product. There is also no need to place the item in a liquid acidic environment or resort to high heat.
Preparing the surface for chrome plating is similar to the process before applying paint. Thanks to this, mirror coatings can cover any base, but it is better if they are metallic. Such chemical treatment does not require significant cash injections. It is enough to purchase a special installation and reagents. As a result, the owner of the equipment will be able to apply a “silver mirror” even to porous or organic materials. No other technology can produce similar results. Today, chrome plating is a powerful competitor to other metallization processes.
Treatment of coatings after metallization
The coating obtained after spraying is largely porous in structure. Its porosity can be effectively used in some cases. Pores can be filled by applying a layer of paint to the coating or impregnating the coating with special compounds. However, dense coatings are most widely used.
Advantages of electric arc metallization:
- high anti-corrosion resistance of metallization coatings;
- no deformation of products;
- mobility of metallization installations and the possibility of applying protective coatings in the field;
- high process productivity;
- high adhesive strength of metallization coatings (in comparison with paint and varnish or flame coatings);
- high plastic characteristics of metallization coatings.
The main disadvantages of metallization are large porosity (up to 20%) and significant losses of metal during sputtering.
Application area
Electric arc metallization, together with the subsequent application of paints and varnishes to metal structures, refers to hybrid coatings, the service life of which, due to the synergistic effect, significantly exceeds the total service life of each of these layers.
These coatings are designed for long-term anti-corrosion protection of metal structures, which during operation are exposed to aggressive environmental factors both outside and inside buildings, as well as in liquids.
Coatings created by electric arc metallization have found application in corrosion protection systems:
- metal structures;
- reinforced concrete supports (bridges, overpasses, overpasses);
- pipelines, fuel and oil storage facilities;
- technological equipment for oil production and petrochemical production, heating networks.
Mechanical processing of coatings
When it is necessary to obtain a clean surface with exact dimensions and a given roughness, the coating sprayed with a certain allowance is subjected to mechanical processing. The main types of mechanical processing of coatings are cutting and grinding. To process coatings made of carbon and corrosion-resistant steels by turning or milling, you can use high-speed cutting and carbide tools. Grinding of coatings can be carried out either with or without the supply of coolant. “Wet” grinding is preferable in cases where there are no problems associated with the penetration of coolant into the pores of the coating. Rough sanding (whether dry or wet) can cause cracks in the sanded surface. Therefore, in order to obtain a high-quality surface, it is necessary to choose the right grinding wheel and grinding modes. Typically, wheels with a relatively coarse structure and a weak bond are used for grinding applied coatings.
After final sanding, the surface of the coating should have a matte shine and contain small pores. A surface that is too shiny and lacks pores indicates improper sanding.
Compaction of the coating and filling of its pores with sealing materials, when necessary, is carried out before grinding. Sealing materials prevent particles of abrasive materials used during grinding from penetrating into the pores of the coating. If the pores of the coating are not filled with sealing materials, then after grinding it is necessary to rinse the coating and remove particles that got into it during grinding. This is especially important for coatings applied to bearing surfaces. Coatings made of soft materials (tin, zinc, babbitt) can be honed, resulting in a smooth surface with little porosity.
Technological process
Vacuum metallization, based on the evaporation and precipitation of metal particles onto the substrate, is a series of sequential processes. They are quite complex.
When metal is heated, it undergoes a number of changes before becoming a coating. First, it evaporates, then is adsorbed, after which it precipitates as condensation and crystallizes on the surface, forming a metal film. Each process is quite complex.
The quality of the finished product is influenced by many factors. The main ones are the physical and technical characteristics of the workpiece materials and the maintained conditions of the metallization process. The formation of the coating layer occurs in two main stages. This is the transfer of mass and energy from the source and their uniform distribution over the surface of the workpiece.
What surfaces can it be applied to?
In general, any materials that are resistant to heating up to +80 and the effects of special varnishes can be metalized in this way. And also, the materials should not be porous, so that during the metallization process in the vacuum chamber no atmospheric or other gas is released, which will lead to poor-quality coating. These include poorly processed ceramics, wood, and concrete. But even decorative coatings can be applied to them in this way, if they are first primed with special compounds.
Most often today, objects made of plastics and metals are processed in this way. This process only enhances their positive properties. Spraying is applied to metal surfaces of products consisting of various alloys. At the same time, protection against corrosion is created, the electrical conductivity properties of the metal change upward, and the appearance of objects improves.
Metallization of plastics makes it possible to produce beautiful, practical products from cheap raw materials. In the automotive industry, plastic parts are installed to reduce weight. Radiator grilles, housings, wheel caps and other parts that do not require increased strength are made from durable plastics and processed to resemble metal.
This technology, like other equally complex ones, has its pros and cons:
- the need to use expensive equipment,
- high energy costs,
- the need for a spacious production facility to accommodate all devices and for the full technological manufacturing cycle.
Additional costs are required for the technical process of applying an additional layer - protective varnish.
Vacuum deposition installations are a set of devices that consistently and independently perform a number of functions necessary for the metallization process.
Main functions:
- pumping out air to obtain vacuum conditions,
- spraying metal particles onto the surface of objects under certain conditions,
- transportation of processed parts,
- control of the modes of ongoing vacuum deposition processes,
- power supply and other auxiliary devices.
Components of a vacuum installation:
- Working chamber. The metallization process itself takes place in it.
- A source of evaporated metals along with control and energy supply devices.
- Monitoring and control systems for adjusting temperature, deposition speed, film thickness, and its physical properties.
- Pumping and gas distribution system, providing vacuum and regulating gas flows.
- Working unit blocking systems, power supply units.
- A transport device that determines the supply and removal from the vacuum chamber, changing the positions of parts when applying a metal coating.
- Auxiliary devices – dampers, in-chamber manipulators, gas filters, etc.
Equipment Features
Installations for the vacuum process of applying a metal layer are magnetron and ion-plasma. In any of them, it is necessary to achieve evaporation of the substance from the surface of the metal blanks, bypassing the stage of molten metal.
With the sublimation method, the heating process occurs quickly to the evaporation temperature, preventing melting. For this purpose, heaters are used that can increase kinetic energy up to the destruction of the crystal lattice. But some metals do not sublimate in a vacuum, and therefore the melt stage cannot be avoided with them. Therefore, in such cases, additional filter systems are used.
Using the method of vacuum deposition of a metal layer, products of different sizes are coated: large (up to 1 m) and very small. There are technologies for metal coating of multi-meter fabrics and films - they are rewound from one roll to another during the deposition process in a vacuum chamber. Therefore, there are installations with working chambers of different sizes:
- small - a few liters,
- large - several cubic meters.
Filler materials
- solid section;
- powder
The flow rate is prescribed at 220–850 m3/h.
To create a protective layer of metal elements with their subsequent seating or for a fixed connection, a solid wire thread is used. To create surfaces of increased hardness during electric arc metallization, powder rods should be used.
To form anti-corrosion layers, highly alloyed iron-based filler materials and non-ferrous metal wires are used.
For application by electric arc metallization, aluminum, zinc and compounds based on them are most often used.
Aluminum is an active substance, but under the influence of oxidizing agents a protective film is formed on its surface, minimizing the ability for chemical interactions. Aluminum's resistance to corrosion varies depending on operating conditions. In a polluted environment, corrosion develops more intensely.
Chemical reagents used
Chemical metallization technology involves the use of various substances that bind together to form a protective coating after undergoing a chemical reaction. Using an activator and reagents for chemical metallization, you can do without special equipment, but the method is not suitable for large parts.
To carry out the processing in question you will need:
- The reducing agent is the main component. Chemical metallization reagents should be stored according to the recommendations provided by the manufacturers.
- The activator is also an important reagent that determines the performance of the surface. Chemical metallization reagents have labels that indicate the name of the metal. Let's take gold, mel and chrome as an example.
- The primer is applied to the surface to ensure the most favorable processing conditions. It significantly increases the adhesion of the applied metal.
- The varnish protects the applied coating from chemical and mechanical influence.
- In order to give the surface a certain color, special toners are used. The specific shade is indicated on the toner packaging.
Reagents for chemical metallization
It is worth considering that when doing the work yourself, it is quite difficult to ensure high quality surfaces. In some cases, you have to use special cleaning compounds.
Considering the disadvantages of chemical metallization, we note that when carrying out this procedure, harmful chemicals are used, work with which must be carried out in strict compliance with safety precautions. This technology is quite simple to implement and resembles the method of coating a surface with a paint and varnish substance.
What is the advantage of chrome plating plastic?
The main advantage for the chrome plating stage of plastic products is the ability to impart shine and an aesthetic appearance to the product, which will completely imitate metal.
Plastic elements that have undergone chrome plating have high levels of withstanding mechanical loads, for example, this is important for plastic machine parts. The chromium layer on the plastic increases the density and strength of the material in case of constant use of the product. The constancy of characteristics at the stage of metallization allows maintaining original characteristics due to the influence of high temperatures, as well as during sudden temperature changes, up to negative values. The wear resistance of the surface will have high parameters due to the properties of hard chromium. The appearance of the plastic elements will have an attractive aesthetic appearance and will not cause any complaints from others.
Thus, high-quality chrome plating of plastic will not cause defects, scratches, or obvious damage to the finished product.
Technological process
Vacuum metallization, based on the evaporation and precipitation of metal particles onto the substrate, is a series of sequential processes. They are quite complex.
When metal is heated, it undergoes a number of changes before becoming a coating. First, it evaporates, then is adsorbed, after which it precipitates as condensation and crystallizes on the surface, forming a metal film. Each process is quite complex.
The quality of the finished product is influenced by many factors. The main ones are the physical and technical characteristics of the workpiece materials and the maintained conditions of the metallization process. The formation of the coating layer occurs in two main stages. This is the transfer of mass and energy from the source and their uniform distribution over the surface of the workpiece.
Sulfate electrolytes
Sulfate electrolytes are simple in composition and very stable in operation. However, the standard electrolyte used in electroplating production, containing copper sulfate (200-250 g/l) and sulfuric acid (50-75 g/l), has poor dissipation ability (<20%) and poor copper deposit quality. In the production of printed circuit boards, to increase the dissipative ability of the electrolyte and increase cathodic polarization, electrolytes diluted in copper sulfate and concentrated in sulfuric acid are used. To obtain compact, shiny deposits, leveling and shine-forming additives and always a wetting agent are introduced into the electrolyte composition. In such an electrolyte, copper is present in the form of divalent Cu2+ ions. However, in the presence of metallic copper, along with Cu2+ ions in the electrolyte, Cu+ may also be present in small quantities due to a disproportionation reaction:
With increasing temperature and decreasing acidity, the equilibrium shifts towards the formation of Cu+, which contributes to an increase in these ions. When the concentration of Cu+ in the solution is greater than the equilibrium one, metallic copper can be released in the form of a fine powder.
If the acidity of the solution is insufficient, the cuprous salt easily undergoes hydrolysis to form copper (I) oxide:
As a result, the electrolyte becomes contaminated with suspended particles of powdered copper or Cu20, which, moving to the cathode, are included in the coating composition; the quality of the copper deposits on the cathode deteriorates - they turn out dark, loose, and rough. The formation of roughness in a freshly filtered electrolyte is a consequence of this phenomenon.
In the presence of a sufficient amount of acid, Cu2S04 is oxidized by atmospheric oxygen to form CuS04:
and thus one of the reasons for the roughness of copper deposits is eliminated.
In this case, the concentration of sulfuric acid decreases and the solution is enriched with copper sulfate. Sulfuric acid in the copper plating electrolyte is necessary:
1) to prevent the accumulation and hydrolysis of monovalent copper;
2) to increase the electrical conductivity and dissipative ability of the electrolyte;
3) to reduce the activity of copper ions, which contributes to an increase in cathodic polarization and the formation of fine-grained deposits on the cathode.
Electrode processes consist of Cu2+ discharge at the cathode and copper ionization at the anode. The Cu2+ discharge proceeds in two stages according to the scheme: Cu2+ -> Cu+ -> Cu, and the slow stage in the cathodic process is the addition of the 1st electron:
DIY chemical metallization at home
If this is your first time performing chemical metallization at home, then you should study theoretical materials in advance and watch videos on this topic. Of course, you need to prepare equipment and supplies. In the process of chemical metallization, hazardous chemicals are used, which must be handled very carefully and comply with safety requirements. Chemical metallization itself, as mentioned above, is not a complicated method, and it can be easily carried out at home. Despite all the advantages of the method, we should not forget that even at home, chemical metallization is quite expensive. The price of a factory coating is even higher and will depend on the surface area covered.