Most often, powder mixtures of the following compositions are used:
- 49.5% aluminum + 49.5% Al2O3 + 1% NH4Cl;
99% ferroaluminum + 1% NH4Cl;
In all compositions, the aluminizing temperature is maintained at 950...1050 °C, and the holding time for the indicated temperatures is set in the range from 6 to 12 hours. Under such conditions, the depth of the aluminized layer can be 0.25...0.6 mm.
The aluminizing process itself is carried out as follows. Parts and powders are loaded layer by layer into iron or nichrome boxes. During the saturation process, 10-15% of fresh powder mixture is repeatedly added to them. If the mixture contains aluminum oxide, it is first calcined at a temperature of 800-900 °C before loading it into the box. All components of powder mixtures are sifted through a sieve with a mesh size of 0.4-0.5 mm. The box in which aluminizing is carried out must be equipped with a fusible shutter. Along with the parts, two or three control samples, called witnesses, are placed in the box. With the help of such witnesses, you can monitor the progress of saturation.
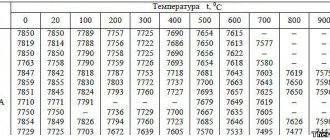
Figure 1 shows the dependence of the depth of the aluminized layer on steel 10 on the duration of saturation at various temperatures. Aluminizing in molten aluminum.
The essence of this method is to soak the parts in a bath of molten aluminum at temperatures of 720 -850 °C. Since some parts can dissolve in liquid aluminum, to prevent this process, 8-12% iron is introduced into the bath. It is undesirable to contain impurities of copper, zinc and silicon in the bath, because they make the process of saturation difficult.
The holding time may vary depending on the type of parts and their purpose from 15 minutes. up to 1 hour Under such conditions, it is possible to obtain aluminized layers with a depth of 0.1...0.3 mm. It should be noted that with this method, an increase in the fragility of the resulting layer can sometimes be observed. Therefore, in order to eliminate such a defect, parts after aluminizing are subjected to annealing at a temperature of 950-1050 °C for 4-5 hours. With such heat treatment, the layer depth can increase by 20-40%.
During the aluminizing process, it is recommended to create a layer of flux on the surface of the melt, consisting, for example, of 40% NaCl; 40% KCl; 10% Na3AlF6; 10% AlF3. This flux plays the role of protection and reduces the process of corrosion of the surface of the part. In Figure 2
The dependence of the layer depth of steel 10 on the duration of aluminizing in the aluminum melt at different temperatures is shown.
The aluminized layer is a solid solution of aluminum based on the chemical compound Fe3Al. This phase is more often called the ?-phase. The aluminum concentration in this phase can reach up to 30% or more.
Currently, aluminizing by metallization is gradually developing and expanding. The essence of this method is to spray a layer of aluminum onto the surface of the part, followed by diffusion annealing at a temperature of 900-1000 °C. Before annealing, the part is coated with a coating consisting of 48% silver graphite, 30% quartz sand, 20% clay and 2% ammonium chloride. All components are mixed on liquid glass and applied to the part with a thickness of 0.8-1.5 mm.
The temperature at which saturation occurs is 900-950 °C. The process itself can last 2-4 hours. Under such conditions, it is possible to obtain a layer with a thickness of 0.2-0.4 mm. Aluminizing is most widely used in the production of valves for internal combustion engines, thermocouple covers, etc. In principle, aluminizing can be prescribed for any parts that operate at high temperatures and which, above all, are required to have high scale resistance.
Aluminizing steel
Over the course of several centuries, the basic performance properties of metals have been changed using chemical-thermal effects. Tests indicate that the percentage of certain impurities in a metal can affect its hardness, strength, corrosion resistance and many other qualities. Aluminizing carbon steel is the process of saturating the surface layer of a product with aluminum, which takes place at a certain temperature. The process of aluminizing steel is quite complicated; it requires the installation of certain equipment. Let us consider the features of the work on saturating the surface layer of steel and cast iron with aluminum.
Aluminized metals and alloys
Aluminizing is not only a way to protect the surface. Oxide film is an excellent base for paint and varnish coatings. The main metals that are subjected to aluminization are:
- Carbon steel. With a high carbon content in the metal, the diffusion of aluminum is difficult, so low- and medium-carbon steels are mainly processed.
- Alloy steel. Processing this metal is associated with certain difficulties, however, if all technological requirements are met, a wear-resistant protective layer can be obtained.
- Cast iron. Cast iron is processed less frequently. The goal is to change the physical properties of the surface layer of cast iron.
The manufacturing process of stainless steel involves aluminizing alloy or carbon compounds.
In addition to the above metals, a protective layer is applied to the following materials:
- copper;
- titanium;
- molybdenum;
- nickel;
- niobium.
Aluminizing methods
Aluminizing steel is performed at temperatures from 700 to 1100 °C, depending on the characteristics of the workpiece. There are several methods of surface aluminization:
- in powder mixtures (calorization);
- spraying;
- metallization;
- in a vacuum;
- by immersion.
Each method has advantages and disadvantages. The technical characteristics of the layer will also have different parameters.
Aluminizing steel by immersion is the most preferred method.
Properties and advantages of aluminized steels
Aluminized steel has a number of valuable qualities:
- After chroming, a surface with high adhesion to paint and varnish products is obtained.
- The low cost of coating allows the use of aluminizing as a worthy alternative to expensive heat-resistant coatings.
- Aluminized steel is resistant to mechanical damage.
- At temperatures above 470 °C, an intermediate alloy is formed, which has high resistance to temperature influences.
Laboratory tests have shown that, with equal thickness, the aluminum layer is 2.5 times stronger than zinc.
Aluminizing is a high-tech process that gives the surface of the metal being processed new protective properties. What do you think about technology? Perhaps you think that there are better methods of metallization? Share your thoughts in the comments section.
Purpose of the process
Normalization is designed to change the microstructure of steel; it does the following:
- reduces internal stress;
- through recrystallization, it refines the coarse-grained structure of welds, castings or forgings.
The goals of normalization can be completely different. Using this process, the hardness of steel can be increased or decreased, the same applies to the strength of the material and its toughness. It all depends on the mechanical and thermal characteristics of the steel. Using this technology, it is possible to both reduce residual stresses and improve the degree of machinability of steel using one or another method.
Steel castings are subjected to this treatment for the following purposes:
- to homogenize their structure;
- to increase susceptibility to heat hardening;
- to reduce residual stresses.
Products obtained by forming are subjected to normalization after forging and rolling in order to reduce the heterogeneity of the structure and its banding.
Normalization together with tempering is needed to replace the hardening of products with complex shapes or with sharp changes in cross-section. This will prevent defects.
This technology is also used to improve the structure of the product before hardening, increase its machinability through cutting, eliminate the secondary cement network in hypereutectoid steel, and also prepare the steel for final heat treatment.
Process technology
Preparation, nitrogen saturation and finishing of the top layer of steel and alloys involves several steps:
- Preparatory heat treatment of metal, which consists of hardening and high tempering. At the same time, the inside of the product becomes more viscous and durable. Hardening takes place at a very high temperature of about 940 ° C and ends with cooling in a liquid - oil or water. Temperature conditions for tempering are 600-700 °C, which gives the metal a hardness suitable for cutting;
- Mechanical processing of workpieces, which ends with grinding. After this procedure, the part reaches the desired size;
- Precautionary measures for those parts of products that must be exposed to nitrogen saturation. For this, simple compounds like tin or liquid glass are used, applied in a layer of no more than 0.015 mm by electrolysis. Occurs by the formation of a thin film impermeable to nitrogen;
- Nitriding of steel using the technology described above;
- Finishing parts to the required condition.
, complex-shaped workpieces with thin walls are strengthened at 520 ° C.
Regarding the change in the geometric parameters of products after the nitriding process, it is noted that it depends on the thickness of the resulting nitrogen-saturated layer and the temperatures applied. However, this change is insignificant in any case.
It should be noted that modern methods of metal processing by nitriding are carried out in shaft furnaces. The maximum temperature of which can reach 700 °C, the circulation of ammonia in such furnaces is forced. The muffle can be built into the furnace or replaceable.
The process will go much faster if you introduce an additional muffle. Then the spare muffle with parts is loaded immediately when the first one with processed workpieces is ready. However, the use of this method is not always economically justified, especially when large products are saturated with nitrogen.
What is more expensive: aluminum or stainless steel Metalworker's Guide
The heating season has ended with grief, after which the issue of changing batteries came to the fore. It’s time to retire the leaky ancient cast-iron radiators to a well-deserved rest, replacing them with something more modern.
Private developers, when installing heating, also often cannot decide on the type of radiators. After listening to salespeople in stores praising the most popular models, an ignorant buyer is at a loss.
And he still has no idea which radiators are better - aluminum or bimetallic. Perhaps let's look at this issue objectively?
The fins located on the inside can significantly increase the heat transfer area to 0.5 square meters. Radiators are made using two methods.
The extrusion method produces cheap and lightweight products that are not of the highest quality (this method is not used in Europe). Radiators made by casting will be more expensive, but more durable.
One of the types of aluminum radiators.
2. Bimetallic radiators are made from two different metals. The body, equipped with ribs, is made of aluminum alloy. Inside this housing there is a core of pipes through which coolant flows (hot water from the heating system). These pipes are made either from steel or copper (and the latter are practically never found here). Their diameter is smaller than that of aluminum models, so they are more likely to clog.
The appearance of the bimetallic radiator is very aesthetic, and the design satisfies the most sophisticated needs. All its steel components are hidden inside.
Purpose
Normalizing steel has different functions other than increasing its hardness. In some cases, normalization is carried out with the opposite purpose to reduce strength and toughness.
The main goals of metal normalization include:
- Obtaining the result of stress leveling. After processing, the steel has additional parameters, which makes it easier to process it in different ways.
- Reducing the heterogeneity and banding of the structure. In this case, objects are subjected to normalization after forging or rolling using the pressure method.
- Reducing the risk of deformation of parts that have sharp differences in cross-section or a complex configuration.
- Changing the coarse-grained structure of steel to fine-grained. Normalization helps to remove the network of secondary cement in hypereutectoid steel, improving its ability to be processed and hardened.
Sulfocyanation
This treatment is more reminiscent of the cyanidation process. The surface is saturated not only with carbon and nitrogen, but also with sulfur. Sulfocyanated parts have, to a greater extent, the same characteristics as cyanidated parts. Sulfocyanated parts performed best in mechanisms under medium loads. Thanks to a slightly different cyanidation scheme, seizing and enveloping of the metal is prevented.
The normal melting point of the mixture is 560-580 degrees. The processing of steel alloys is carried out mainly in liquid media, but it is also possible in gas media. Since sulfocyanated parts have a little more strength, their use is justified as piston rings, cast iron bushings, and various pump spare parts.
Types of metal heat treatment
There are 3 main types of metal heat treatment:
- annealing;
- hardening;
- vacation.
There is also thermochemical treatment, which refers to combined methods of imparting properties of increased hardness and wear resistance to the material.
Annealing
The essence of annealing is that the metal is heated to a certain temperature, held for the required period of time, and then slowly cooled to normal room temperature.
Most often, annealing is performed to solve the following problems:
- increase in mechanical properties of the material;
- bringing the material to a homogeneous state;
- improved plasticity;
- increasing the level of resistance;
- reducing the internal resistance of the material for subsequent forging.
Annealing is a process that is divided into several types, depending on the nuances of the procedure:
- diffusion;
- complete or incomplete;
- spheroidization;
- isothermal;
- normalization.
There are more annealing methods, but these are the main and most commonly used.
Also, the full annealing procedure involves improving the properties of the material for processing and getting rid of internal resistance. Full annealing is used to process:
- steel with a minimum amount of carbon;
- hypoeutectoid alloy.
In the full process, the product is brought to a critical temperature (point A3) and after the required period of time is cooled to room temperature. Since the specific temperature parameters depend on the type of materials used. As a result, the holding time also directly depends on the type of alloy undergoing this technological process.
With incomplete annealing, the ultimate goal is different - to create, if possible, a softer and more ductile material. In this case, the heating temperature can reach 770 degrees. Cooling is divided into 2 stages: first in the oven, and then in the open air.
An isothermal type of annealing is used for high-chromium steels. This method saves significant production time because one of the cooling stages uses an accelerated process. There is no need to wait until the steel cools down with the furnace.
Metal hardening
During hardening, the product is heated to critical levels. As a result, subsequent cooling is not carried out gradually and naturally, but abruptly and forcibly. In this case, to reduce the temperature, substances such as compressed air, water mist, and liquid polymer quenching medium are used. in addition to strength, the metal receives lower parameters of viscosity and elasticity.
Hardening methods:
- Using one medium is a simple method, which, however, has limitations on the material used. Rapid cooling occurs and temperature unevenness occurs. Metal with a high carbon content cannot be processed in this way, since such material can be destroyed by aggressive influence.
- Multi-stage hardening - first the metal is heat-treated, and after reaching the required temperature it is placed in a salt bath. The temperature is equalized and only then is the material cooled using oil, air or fog.
- Light hardening. With this method, the material is first kept in a salt bath with the addition of sodium chloride. Then it is cooled in a bath with caustic sodium and caustic potassium.
- Self-vacation. With this method, the part is pulled out of the cooling system before the temperature drops. At this time, a high temperature will still remain in the center of the workpiece or part. After the tempering of the part is completed, it is cooled completely by immersion in a special environment.
- Isothermal hardening. An analogue of step hardening with a longer exposure time in a salt bath.
Diffusion metallization of ship parts
Diffusion metallization is usually called a method of processing steels or other metals and alloys, in which the surface layer is changed by the introduction of molecules of other elements.
All this happens at very high temperatures in a special environment.
The result of this treatment is the physical strengthening of the layer, as well as increasing its heat resistance, increasing resistance to the corrosion process - the surface wears less during operation.
Unlike nitrocarburization and cyanidation, where carbon and nitrogen atoms are directly introduced into the crystal lattice of steel, diffusion metallization involves a more complex process when atoms of other elements form so-called substitution solutions with steel, therefore this process is lengthy and requires the use of higher temperatures exceeding 1000 degrees Celsius.
Description of the technology and its purpose
The diffusion metallization method makes it possible to obtain a processed steel layer thickness from 10 microns to 3 millimeters. Regardless of what metal the surface layer of the carrier is saturated with, the production technology has several similar stages:
- Physical cleaning of a part that will be subject to diffusion metallization. At this stage, any dirt, dust, grease and oxide layer are removed from the surface.
- Placing the product in the work environment. This can be dipping into a liquid melt, filling with metal-containing powders, or spraying metal onto the surface of the part.
- In some cases, as with aluminum diffusion, there is a stage of applying a heat-resistant coating on top of the melt sprayed onto the workpiece.
- Placed inside a special oven where high temperatures are created, sometimes exceeding 1000 degrees Celsius. At this stage, under the thermal influence of the furnace, atoms penetrate into the surface layer of the product, which can last for a very long time.
- After processing, the part is removed from the box, washed, and the remaining powder is removed.
In order for the workpiece to have a good appearance (especially for metallization of decorative elements), it should be subjected to additional processing by mechanical polishing.
Diffusion metallization of ship parts
Diffusion metallization is the saturation of the surface layer of a part with any element to impart certain properties to the surface. The most common types of diffusion metallization are:
- aluminizing - saturation with aluminum to increase heat resistance;
- chrome plating - saturation with chromium to increase corrosion resistance, heat resistance, hardness and wear resistance;
- siliconization - saturation with silicon to increase corrosion resistance and acid resistance;
- sulfidation - saturation with sulfur to increase extreme pressure properties and wear resistance;
- phosphating - saturation with phosphorus to improve run-in and wear resistance.
Container ship Alasa ipspotting.com
Saturation is carried out to a depth of 0.3-0.9 mm, keeping the heated part (on average to a temperature of 500-600 ° C) in an appropriate environment for a certain time (several hours).
Recently, complex thermochemical methods for processing parts have been used:
- sulfocyanation;
- chromosiliconation (saturation with chromium and silicon);
- borosilication;
- boronation (saturation with carbon and boron);
- chromonitriding, etc.
Such coatings have high surface hardness and high wear resistance.
New methods of thermochemical processing have appeared:
- ionic;
- energy-releasing pastes.
The first methods are based on the highest activity of the gas, which manifests itself in the ionized state - ion nitriding, ion cementation, etc. Ionic thermochemical treatment is carried out in a hermetically sealed chamber in an atmosphere of a glowing arc or spark discharge.
The essence of ionic methods can be traced, for example, to ion nitriding. The part is placed in a chamber from which air is pumped out. The chamber is filled with ammonia gas and an electrical discharge is generated.
In this case, the electrodes are the anode and the part is the cathode. Ammonia dissociates, breaking down into nitrogen and hydrogen ions.
The electric field accelerates them, ions begin to bombard the surface of the part. Methods for strengthening and increasing the durability of parts, and nitrogen quickly saturates the surface layers.
Container ship Aurora, North Sea ipspotting.com
In the second method, the part is coated with an energy-releasing paste, which is set on fire. When the paste burns, the surface of the part becomes very hot (up to 600-800°C), and the dating elements contained in the paste penetrate into the upper layers of the part. After 2-3 minutes, the burnt part is immersed in water to cool.
With the help of energy-releasing substances in ship conditions it is possible to produce:
- aluminization;
- boriding;
- carbonitriding and other types of thermochemical treatment.
Types of diffusion metallization
The types of diffusion metallization can be classified according to several criteria. First of all, according to the type of metal that will penetrate into the surface layer through diffusion. Here they highlight:
- Aluminizing, when a part is saturated with aluminum atoms by thermochemical method.
- Chromium plating is the diffusion saturation of steel with chromium atoms.
- Titanization is the introduction of titanium atoms into the surface layer of steel.
- Galvanizing, when a metal part is saturated with elementary particles of zinc using a thermochemical method.
- Siliconization is the diffusion saturation of steel with silicon.
- Boriding is the production of a high-strength surface layer of metal by introducing boron atoms there by diffusion.
Depending on the state of the environment where the metal is processed by the diffusion method, metallization is carried out:
- in a solid environment;
- in a liquid medium;
- in a gaseous environment.
Solid metallization
This type of metallization is carried out through the use of an active solid medium based on ferroalloys.
This category includes ferrosilicon, ferroaluminum, ferrochrome (the listed components are introduced into the work area as powders), plus ammonium chloride (NH4Cl) is added to them, not exceeding 5% of the total mass of the solid component. The parts covered with powder are placed inside a special oven.
Saturation in a solid medium is carried out for steel, cobalt, nickel, titanium and other metals at temperatures from 1000 to 1500 degrees Celsius.
When the temperature rises to the operating level, ammonium chloride begins to react with the ferroalloy, resulting in the release of unstable thermal metal chlorides CrCI2, AlCI3, SiCI4 and others. These chlorides, in contact with the steel surface, begin to dissociate. A chemically active element is released, which penetrates the surface layer of the product, saturating it.
Essentially similar heat treatment processes
In addition to normalization, the following operations can be added to the list of heat treatment of steels:
- annealing;
- vacation;
- hardening;
- cryogenic treatment and several others.
The annealing operation provides a high-quality, finer structure of pearlite; this occurs because furnaces are used to cool the parts. The purpose of this operation is to reduce the heterogeneity of the structure, remove stress, and increase machinability.
The principles underlying the hardening operation are identical to those of normalization, but there are some differences. For example, when hardening, much higher temperatures and high cooling rates are used. Hardening leads to improved strength characteristics, hardness, etc. But often workpieces that have undergone hardening are characterized by reduced viscosity and high fragility.
Tempering of parts is used after the hardening operation. Tempering reduces brittleness and internal stress. In this case, the temperature range is lower than that used in normalization. The parts are cooled in air. As the temperature increases, the tensile strength and hardness decrease and at the same time the impact strength increases.
Properties of plasma spraying
When working with metal structures, sometimes it is necessary to supplement them with additional properties so that they can be used in any field.
This way the surface will become even more resistant to moisture, high temperature and chemicals.
Diffuse metallization has many features that make it unique among other types of metal processing.
- Due to exposure to high temperatures (five to six thousand degrees), the surface treatment procedure is greatly accelerated. The process itself takes place in a fraction of seconds, and the result is excellent.
- The result is a combined ball. You can apply not only metal elements, but also gas particles from a plasma jet. Thus, the metal surface is coated with atoms of certain metal elements.
- If you carry out classical metal spraying, then the application occurs unevenly, takes a very long time and involves oxidative processes. But with the help of hot plasma, the correct temperature and pressure are obtained, due to which a high-quality coating is formed.
- The plasma jet transports particles of metal and gases at the speed of light, so you won’t even understand anything. Thus, welding occurs with powders, rods, rods and wires. Afterwards, a layer of several microns to one millimeter is formed on the base of the structure.
Steel carburization technology, its essence and purpose - methods and videos
Depending on the specific application of various metals and alloys, additional processing is often carried out. This allows you to highlight (strengthen) certain properties of the sample. What is steel carburization, why is it needed, in what cases is it advisable to carry it out - the reader will learn about this in an accessible form from this article.
There are various methods of chemical-thermal treatment of materials. One of them is cementation. This technology is used for low-carbon and alloy steels, the content of element “C” in which does not exceed 0.25%.
The purpose is to increase such characteristics of the alloy as wear resistance, strength, and hardness.
For implementation, special furnaces are most often used, where the process takes place at high temperatures - about 945 (±15) ºС.
Depending on the dimensions and design features of the product, it is kept in such conditions for several hours. In essence, this is a complex treatment of a part (chemical + thermal) in order to give it hardness.
Pastas
The technology is the simplest, but not always applicable. For parts that have a complex configuration, with various protrusions, grooves, and the like, it is clearly not suitable.
The technique is the surface application of cement paste onto the sample. Its layer is chosen to be large compared to the calculated depth of carbon penetration into the steel (about 7 times).
Conditions - the temperature regime is set depending on the type of paste, ranging from 900 to 1,000 ºС.
Such carburization of steel can be carried out at home, if you have a drying cabinet with the required parameters.
Gas environment
One of the most effective techniques that is widely used in industry. It significantly simplifies the carburization process, reduces steel processing time and increases productivity. The main condition is to choose the right mixture according to the carbon content and the optimal temperature regime.
Methodology - products are loaded from a carburizing furnace into which gas is supplied.
Fluidized bed
This method is only partially reminiscent of the previous one.
Methodology - so-called corundum is placed in the furnace, on the gas distribution grid. Endogas (a mixture into which methane is introduced) is supplied from below and, rising, dilutes it, as a result of which the smallest fractions begin to move along with the flow to the product being processed. At high temperatures, diffusion of corundum particles occurs, and as a result, saturation of the surface layer of the sample with carbon.
Feature - the degree of cementation is easy to adjust by changing the gas supply. This technology makes it possible to uniformly saturate the steel over the entire area.
Experts recommend using this method, taking into account the costs and low complexity, for small-scale production of blanks.
Solid carburizer
As a saturating medium for this cementation technology, coal, peat or charcoal semi-cokes with granules from 3 to 10 mm are used with the obligatory addition of substances that initiate the process (activators).
Methodology - the samples to be processed are placed in a metal container on a sand seal. They are positioned so that they can be covered on all sides with a layer of carburizer. Therefore, contact of products with the walls of the tank or with each other is not allowed.
Carburization conditions – temperature 925 (±25) ºС. The holding time depends on the layer of the saturating medium. Determined at the rate: per 0.1 mm – 1 hour of heat treatment. The process can be accelerated by increasing the heating to 975 - 980 ºС. This reduces the time of the technological operation, but increases energy costs and reduces the quality of the finished product. A mesh will form on its surface, which will have to be removed.
In some cases this is quite difficult, for example, if the product is characterized by relief.
Surface hardening
Many parts operate under conditions of increased surface wear. Therefore, there is a need to somehow protect this surface. This is achieved by surface hardening methods.
To strengthen a surface means to increase the properties of the surface: hardness, wear resistance, corrosion resistance. If it is necessary to change the properties, this means that the structure of the surface layer must change.
To change the structure, you can use deformation, heat treatment with heating in various ways, changing the chemical composition of the surface, and applying protective layers.
Basically, methods for hardening surfaces can be divided into two main groups: 1) hardening a product without changing the chemical composition of the surface, but with a change in structure.
Hardening is achieved by surface hardening, surface plastic deformation and other methods.
2) strengthening of the product with a change in the chemical composition of the surface layer and its structure. Strengthening is carried out by various methods of chemical-thermal treatment and the application of protective layers.
Methods for changing structure
Among the methods of hardening without changing the chemical composition of the surface, but with a change in its structure, the most common methods are surface hardening and various types of surface plastic deformation (SPD).
In essence, surface deformation is the simplest way in which the strength characteristics of a surface increase. The following principle is used here.
If we recall the strain hardening curve, it turns out that the more we stretch the metal, the more the metal resists, the greater the tensile force Pmax (up to a certain limit, of course). The metal is strengthened both during torsion and compression.
In SPD technologies, the surface layer of the metal is deformed (hardened) in various ways. The main purpose of SPD is to increase fatigue strength by hardening the surface to a depth of 0.2-0.4 mm.
Varieties of PPD are shot blasting, roller processing, needle milling, relief rolling, etc.
Shot blasting is the treatment of the surface of finished parts with shot blasting. Used to harden parts and remove scale. Products such as springs, leaf springs, chain links, tracks, liners, pistons, and gears are subjected to shot blasting.
When processing with rollers, deformation is carried out by pressing a hard metal roller onto the surface of the workpiece. When forces on the roller exceed the yield strength of the material being processed, hardening occurs to the required depth.
Roller processing improves the microgeometry of the product.
The creation of residual compressive stresses increases the fatigue limit and durability of the product. Roller rolling is used when processing shafts, calibrating pipes and rods. In Fig. Figure 1 shows the hardened surface layer of a sample of a 45 steel railway car axle.
The microstructure of the layer consists of deformed grains of ferrite and pearlite. Rolling with a roller refined the structure; in the surface layer, individual grains are indistinguishable (Fig. 1, a). Where the deformation was less, one can discern a structure that has a directionality characteristic of deformation (Fig. 1, b).
The depth of hardening is controlled by changes in microhardness (Fig. 2).
| A | b |
Figure 1. Microstructure of the surface layer of steel 45 after rolling with a roller
Figure 2. Variation of microhardness along the depth of the cross section of shafts of different diameters.
Needle milling using cutters, on the surface of which there are from 200 thousand to 40 million densely spaced needles made of high-strength steel wire with a diameter of 0.2-0.8 mm, also makes it possible to harden the surface of parts.
Needle milling is used for processing flat and cylindrical surfaces, as well as for cleaning parts from scale. During needle milling, a hardened surface layer is also formed (Fig. 3). In this case, the strengthened layer consists of deformed grains of ferrite and pearlite (Fig. 3, a).
On the surface that was processed, traces of the cutter are visible (Fig. 3, b).
| A | b |
Figure 3. Microstructure of the strengthened layer of steel 20ХНР (a), initial state - normalization; surface after needle milling (b).
The essence of surface hardening is that the surface layers of a steel part are quickly heated above the hardening temperature and then cooled at a rate above the critical one. The main purpose of surface hardening is to increase the hardness, wear resistance and endurance limit of the surface while maintaining a viscous core.
Heating, in principle, can be carried out in different ways. In industry, the most common method of surface hardening is induction hardening with heating by high-frequency currents. As a rule, the strengthened layer is already visible during macrostructural analysis (Fig. 4). On the left is an unetched section of the sample. It reflects light more when shooting, so it looks dark.
On the right is the area after etching. The hardened layer is clearly visible.
Figure 4. Fragment of an automobile part; macrostructure
Both macrostructural and microstructural analysis (Fig. 5a) shows that the strengthened zone consists of 2 layers: light at the very surface and then darker. The upper light layer has the structure of quenched martensite (Fig. 5b). Martensite formed when the surface cooled rapidly.
The darker layer is tempered martensite (Fig. 5c). This is the martensite that also formed during accelerated cooling, but remained at an elevated temperature longer, which turned out to be enough for tempering to occur.
The core of the part may contain sorbitol or troostite at different depths (Fig. 5d).
| A | b |
| V | G |
Figure 5. Microstructure of the layer (in Fig. 4) obtained by high-frequency quenching: a – layers of quenched and tempered martensite, b – quenched martensite, c – tempered martensite, d – troostite and martensite in the core.
Methods for changing structure and composition
Methods of hardening with changes in the chemical composition and structure of the surface include chemical-thermal treatment (CHT). It consists in saturating the surface layer of steel with various elements at high temperature.
Depending on the saturating element, there are the following types of chemical-thermal treatment: carburization, nitriding, nitrocarburization (cyanidation), boriding, diffusion metallization (alitization, chrome plating, silicon plating, etc.).
Common to all types of surface hardening is an increase in the hardness of the surface layer. The choice of surface hardening method for a part depends on its operating conditions, shape, size, grade of the selected steel and other factors. The most widely used is carburization - saturating the surface of steel with carbon.
Carburization gives the steel surface high hardness and wear resistance while maintaining a tough and ductile core. Cemented products acquire their final properties after hardening and low tempering.
Cementation is usually carried out on parts made of steels with a carbon content of up to 0.25%, operating under conditions of contact wear and the application of alternating loads: medium-sized gears, bushings, piston pins, cams, car gearbox shafts, individual steering parts, etc. d.
The cemented layer has a variable carbon concentration throughout its thickness, decreasing from the surface to the core of the steel part. Therefore, the structure that is formed during cementation in the surface layer will have a different ratio of pearlite, ferrite and cementite. There are four main zones of a steel product after carburization (Fig. 6):
Rice. 6. Microstructure of carbon hypoeutectoid steel 10 after carburization.
1 – hypereutectoid zone, consisting of pearlite and cementite network (Fig. 7a); 2 – eutectoid zone, which is pearlite (Fig.
7b); 3 – hypoeutectoid zone, in which, as you approach the core, the amount of carbon and pearlite decreases, and the amount of ferrite increases (Fig. 7c); 4 – the original structure of the steel product, unchanged after carburization.
The depth of the cemented layer “h” is taken to be the sum of the hypereutectoid, eutectoid and half of the hypoeutectoid zone, where the amount of ferrite and pearlite is 50%.
| A | b | V |
Figure 7. Structure of zones of a cemented part: a – hypereutectoid zone (cementite + pearlite), b – eutectoid zone (pearlite), c – hypoeutectoid zone (pearlite + ferrite).
Figure 8. Change in hardness in the surface layer after carburization and heat treatment
Nitriding is the process of saturating the surface layer of steel with nitrogen and is most often carried out at temperatures of 500-600 °C.
Nitriding, like carburizing, increases the hardness and wear resistance of the steel surface. Figure 9 shows a series of indentations when measuring microhardness on a transverse section of a nitrided sample.
At the top there is a hardened layer (dark stripe). The diameter of the prints decreases as they approach the surface. The hardness is higher there.
Figure 9. “Track” of microhardness imprints; steel part after nitriding
The nitrided layer is usually white. The layer itself does not change during metallographic etching, and underneath the steel has a structure corresponding to heat treatment (Fig. 10). Figure 11 shows an automobile part and the change in microhardness along different “teeth”.
Figure 10. Nitrided layer on 40KhGNM steel
| A | b |
Figure 11. Automotive part (a) and change in microhardness (b) of its surface layer after nitriding
Currently, plasma and ion-plasma nitriding is widely used. The structure of the surface layer after such treatment is finely dispersed martensite (1), under which there is a transition zone (2); the unchanged structure (3) is located deeper (Fig. 12).
Figure 12. Structure of the surface layer after treatment with nitrogen plasma; U8A steel
Boriding is a process of chemical-thermal treatment, diffusion saturation of the surface of metals and alloys with boron during heating. Boriding leads to a significant increase in surface hardness. Boriding is carried out in powder mixtures by electrolysis.
There is also liquid electrolysis-free boriding, ion boriding and boriding from coatings (pastes). Boridation is most often carried out by electrolysis of molten borax (Na2B4O7). The product serves as a cathode. Saturation temperature 930–950 °C, holding time 2–6 hours.
After boriding, a dense white layer of borides is formed on the surface of the sample (Fig. 13). The white layer consists of intertwined columnar crystals of the composition FeB and Fe2B. The structure of the boride layer is influenced by the composition of the steel. In steel 25KhGT (Fig. 13, a) and in steel 45 (Fig. 13, b) there is a solid solution zone between the boride crystals.
In steel 40X (Fig. 13, c) the layer consists only of extended needles of borides. A zigzag interface is formed between the borated layer and the core.
| A | b | V |
Figure 13. Structure of borated layers in steels 25KhGT (a), 45 (b), 40Kh (c)