The battery is a lattice plate made of either lead dioxide or pure lead, sometimes coated with calcium. Between them is an aqueous solution of sulfuric acid. Lead and acid react with each other to create electricity, but break down into other elements that do not create electricity (salt and water). The battery is dead. When we charge the battery, that is, we apply current to the electrolyte, a reverse reaction occurs: water reacts with salt, forming acid and metal (or metal oxide), which are again capable of creating electricity.
What is battery desulfation?
Sulfation of acid battery plates
Desulfation is the removal of sulfuric acid salts from the battery plates.
Desulfation is the removal of sulfuric acid salts (lead sulfate or calcium sulfate). This salt appears on the walls of the lead plates as a result of a chemical reaction that occurs during battery discharge. However, not all the salt is converted back when charging the battery. Some of it settles on the metal plates, preventing contact between lead and acid, and over time there is so much lead sulfate that the battery stops working altogether.
Possible difficulties
Some difficulties may arise when desulfating power sources. For example, maintenance-free batteries and gel-type batteries cannot be restored, due to the fact that it is impossible to replace the electrolyte in them (although in the first case you can make a hole and drain the liquid composition). However, manufacturers make such batteries less susceptible to sulfation, which significantly extends their service life.
In addition, in calcium batteries the plates consist of an alloy of calcium and lead. And the use of special alloying materials reduced the evaporation of water from the electrolyte solution, which significantly reduced the self-discharge of the battery. During operation of the power source, together with lead sulfate, a coating of calcium sulfate forms on the plates, which does not decompose when the current is applied back or under the influence of chemical reagents.
How to desulfate a car battery
Proper battery desulfation is a method of alternating short, weak charges with short, weak discharges. To carry out such cycles, there are special chargers for car batteries with desulfation. Let's say a few words about the “wrong” (in quotes, because such methods exist, but we do not recommend them) desulfation of battery plates.
- Mechanical cleaning of plates from lead sulfate (we disassemble the battery, take out the plates and clean them).
- Chemical cleaning (open the filler cap, pour in a special solution that will corrode the salt on the lead).
These methods are controversial (in terms of effectiveness) and are very dangerous. But the choice, of course, is yours.
How to desulfate a battery at home
Battery desulfation at home
To desulfate the battery, chargers with a desulfation mode and special devices for this are sold.
As mentioned above, you can purchase a battery charger with a desulfation mode, or a special device for desulfation. In this case everything is simple. We connect the battery to the device and monitor the indicators on the display; sometimes this process can take several days depending on the degree of sulfation. Note that such a device is not cheap and it makes sense to “get confused” to make a device for desulfating the battery with your own hands. First, let's try to do the simplest thing possible. Namely, desulfate the battery with a charger. Before starting work, check the density (usually 1.07 g/cm³) of the electrolyte level in the battery; if it is not enough, then add distilled water (not electrolyte!).
It is very important that after 8 hours of charging the battery at low current, disconnect it from the charger for a day.
- Let's take our regular charger and set the voltage on it to 14 V (but not more than 14.3), and the current to 0.8-1 A (there are chargers that cannot set such parameters, which means such chargers are not suitable for us ). Desulfation of the battery with low current is carried out within 8 hours (some error is allowed, for example, you can leave the battery to charge overnight). We check the density of the electrolyte, it should be approximately the same as at the beginning of the “experiment,” but the voltage should change and be 10 V.
- If everything is so, then we disconnect our battery from the charger for a day (this is important!).
- The next stages of desulfation will be to set the current to 2-2.5 A at the same voltage. We also leave the battery to charge for 8 hours. Then we check the battery voltage (12.7 V) and density (1.11-1.13 g/cm³). If the indicators correspond, then we proceed to the next stage.
Discharging the battery using a light bulb.
This method of restoring the battery will take you from 8 to 14 days, and the battery will be restored by 80 - 90%.
- We connect a low-power consumer of electricity to the battery (for example, a low-beam lamp). We discharge the battery to 9 V, this will take approximately 8 hours. In this case, it is necessary to monitor the voltage in the battery (it should not drop below 9 V), otherwise the process of plate sulfation will start again, which we are trying to get rid of. The density should remain at 1.11-1.13 g/cm³.
- We repeat the previous 4 steps. In this case, the density will increase slightly (1.15-1.17 g/cm³). Then we perform 4 steps again, and again, until the density of the electrolyte is approximately 1.27 g/cm³.
This method of restoring the battery will take you from 8 to 14 days, and the battery will be restored by 80 - 90%.
Preventive measures
To reduce the effect of sulfation, it is necessary to check the density and level of the electrolyte (distilled water is used to top up). The recommendation applies only to power sources equipped with screw caps in the lids of the cans.
Long-term storage of a battery connected to the vehicle's on-board network negatively affects the condition of the plates. The sulfation process intensifies at low air temperatures. An additional preventive measure is to comply with the charging current parameters, which should not exceed 10% of the battery capacity.
Diagram of a device for battery desulfation
Charger circuit for battery desulfation
The basic principle of the “blinker” for battery desulfation is that the charge should be no more than 10% of the battery capacity and the voltage should be within 13.1 - 13.4 V.
In order to restore the battery, you can create a load circuit with your own hands, in which charges will alternate with discharges. Such a circuit consists of relays and 12 V light bulbs. The lamps put a load on the battery and discharge it to a certain limit, the relay, in turn, turns off the circuit at the moment of this limit, and then turns on the “blinker” when the battery is charged again to the required level. The basic principle of the “blinker” for battery desulfation is as follows: the charge should be no more than 10% of the battery capacity and the voltage should be within 13.1 - 13.4 V. The voltage can be monitored manually using a voltmeter connected to the network, or you can connect one more, auxiliary, relay that will control the set voltage. Typically, the ripple mode of the circuit is as follows: for 4.3 seconds there is a discharge with a current of 1 A, then there is a charge of 5 A for 3 seconds. Since the load lamps turn on and off alternately, the circuit seems to “blink”, which is why it got the name among the common people “blinker”.
Desulfation by reverse charging method
It is worth noting that this method is very risky, so when using it, everyone bears personal responsibility. You can hear many conflicting comments about this method from car owners. But if there is nothing left to lose and you don’t mind the battery, then you can take the last chance and try to restore the battery using reverse charging.
The first step is to get a powerful DC source. In this case, the best option would be a welding device, not an inverter one, but a linear one. It is important that it can produce a current of 80 amperes at a voltage of 20 V.
The battery is disconnected from the vehicle's power supply and placed on a flat surface. All the plugs on the cans are unscrewed, and the battery being restored is connected to the welder in the reverse order - minus to plus, and plus to minus.
Then the improvised charger is plugged into the network and the battery is left to charge under such conditions for about 30 minutes. Naturally, the electrolyte will boil, but you should not pay attention to this; after desulfation, it will need to be replaced. If everything goes well, the plates will be cleared of lead sulfate, and the polarity at the terminals will change its value.
Afterwards the battery is disconnected from “charging” and the old electrolyte is drained. The inside of the battery is thoroughly washed with distilled water. Then a new solution is poured in and the battery is charged with ordinary charging in normal mode. When connecting the charger, do not forget that the polarity has changed. The charging current and duration will depend on the battery characteristics, the standard value is 10% of the total battery capacity.
How to desulfate a maintenance-free battery
Homemade battery desulfation device
Desulfating or cleaning the plates from sulfuric acid salts will extend the life of your battery, but, unfortunately, not for long.
A maintenance-free battery cannot be desulfated for the simple reason that there are no filler holes in it, which means it is impossible to check the level and density of the electrolyte. In practice, the battery capacity is examined with a flashlight, the liquid level is determined, a hole is made above this level, and distilled water is added through this hole with a syringe. Upon completion of the work, the hole is sealed. You can also try to restore a maintenance-free battery using a circuit for cyclic discharging and charging, in some cases this helps. A calcium battery can also be classified as maintenance-free, but for a different reason. In such batteries, along with lead sulfate, calcium sulfate is formed (lead plates are doped with a layer of calcium, which gives such batteries a number of advantages), which in turn “plasters” the plates, and subsequently the space between them. If you do desulfate the calcium battery, the calcium sulfate will dissolve along with the coating layer. Let's summarize. What does desulfation give us for the battery? Cleaning the plates from sulfuric acid salts will extend the life of your battery, but, unfortunately, not for long. In any case, if your battery is sulfated, this is a sure sign that it has already exhausted its resource and whether it makes sense to restore the battery is up to you to decide.
Read also: Motor 3 phase 220 volts
Sooner or later, every driver has to face the problem of battery sulfation. When the first signs of sulfation appear, you can leave everything to chance and wait until the battery runs out, then replace it with a new one, or try to extend its life by your actions. There are several ways to do this, by desulfating the battery plates, maintaining its vital activity. In this article, we will consider what the desulfation process is, how to carry it out, and what are the results of such work.
What is car battery desulfation
The concept of desulfation refers to the work of cleaning the battery plates from the lead sulfate that has accumulated on them. Such work is carried out through special charge and discharge cycles.
As you can remember from the definition of plate sulfation, a similar problem occurs during normal operation of a car battery. Over time, the working plane of the positive and negative plates decreases as lead sulfate adheres to them. At the same time, the density of the electrolyte also decreases, down to 1.05-1.07 g/cm 3, which is critically low for normal battery operation.
If the battery plates become coated with lead sulfate, they must be cleaned to ensure the battery continues to perform at its best. To clean the plates, you should use a special charger, with which desulfation is performed.
Please note: If a special device for desulphating the plates is not available, a regular “charger” can be used by performing the special charge and discharge cycles described below. The effect will be worse, but the plates will still be partially cleared of precipitated lead sulfate.
Desulfation involves performing battery charge and discharge cycles using a special technology. Let's take a closer look at how this is done below.
When will it help?
The difference between sulfation and normal battery plates.
Sulfation is not the only cause of battery failure. Before you start desulfation, pay attention to the symptoms for which it will help:
- No physical damage, no deformation of the battery case and current leads, no electrolyte leakage;
- Have not been dropped or overturned;
- The battery charges quickly and discharges quickly (signs that capacity has decreased);
- When charging, the electrolyte boils quickly;
- The case heats up quickly;
- If the battery case is serviceable, that is, it has filler plugs, unscrew them and inspect the plates - a white and light coating is a sure sign;
- The actual capacity of the battery is no more than 50% of the declared one.
Which batteries can be desulfated?
To desulfate a battery, it must meet a number of criteria. When a battery fails, it is not always a sign that its plates have become sulfated. This can happen, among other things, due to the destruction of the plates or the short circuit of the cans. That is, before starting the battery desulfation process, you should make sure that:
- There is no mechanical damage on the battery case, that is, it did not fail as a result of a fall or impact;
- There is a white coating on the battery plates. This can be checked by unscrewing the plugs;
- Checking the battery capacity shows that it is around 30-40%;
- When you try to charge the battery, it takes a charge, but quickly discharges. In addition, it heats up quickly and may boil while charging.
If the cause of the battery malfunction really lies in the sulfation of its plates, you can proceed to the desulfation process.
Some tips on battery recovery techniques
Desulphating a battery is not the only method of restoring its normal performance. It can fail not only due to a large amount of deposits on the plates. Failure can also be caused by constant overcharging of the battery, which leads to corrosion of current-carrying parts and destroys the putty of the plates themselves. Excessive vibration can also cause detachment of the active substance.
There are several important rules for carrying out desulfation, these include the following:
- the process is carried out in a well-heated (20-25 °C) and ventilated room;
- do not allow the electrolyte to boil;
- Do not desulfate more than once a year.
Naturally, the entire process must be controlled.
Desulfation of plates with a special charger
On sale you can find special charging stations designed to desulfate a car battery. They operate in the required discharge-charge mode and cope effectively with the task of cleaning the plates from lead sulfate.
Please note: The cost of a charging station designed to desulfate battery plates is quite significant. For the price of one such station you can purchase 3-4 new batteries. Accordingly, having such a station for “home use” is not economically profitable.
Having a special charging station, it is very easy to carry out work on desulfation of plates. To do this, just take the battery, connect the charging station to it and turn on the desulfation process, after which the device will do everything automatically.
Please note: The desulfation process using a special charger takes several days.
This charging station works very simply. Voltage is supplied to charge, and after a while the discharge begins. Most often, the charging and discharging currents are in a ratio of 10 to 1, that is, if a current of 2 Amperes is supplied to the charge, then 0.2 Amperes are supplied to the discharge.
When the work is completed, the charging station will use an indication to show how much the battery capacity has been restored, if it has appropriate indicators or a display.
Tips for operation and assembly
The requirements for the power transformer are similar to those of the previous circuit. Diode 1VD1, thyristor 1VS2 and triac 1VS1 must be installed on radiators. Powerful resistor 1R11 can be anything (single or multiple), and 1R1 must be a wire resistor. It wouldn't hurt to install a fan in the housing. It can be powered from the +12 volt line. The 1R6 resistor is installed on the front wall of the case, and a voltmeter and ammeter must also be mounted there.
For clarity, we recommend a series of thematic videos.
Desulfation can significantly extend the life of a battery. It can be done at home, and the safest and most effective method is electrochemical. If you have qualifications, you can make a device for performing such a procedure yourself.
Desulfation of plates with a conventional charger
As noted above, work on desulfating the battery plates can be done using a conventional charger. But the work in this case will be much more complex and will require regular intervention in the process.
We will consider the desulfation process for a battery that has a terminal voltage of 8 Volts and an electrolyte density of about 1.07 g/cm 3 . During normal charging, such a battery begins to boil after about 15 minutes, without receiving voltage.
Desulfation is performed using a conventional charger as follows:
- Remove the battery and place it in a well-ventilated area where the work will take place;
- Next, check that there is enough electrolyte in the battery. If it is not enough, then add regular distilled water. Please note that under no circumstances should you add electrolyte or concentrate ;
- Next, you will need to take a regular charger, which allows you to rigidly set current and voltage indicators (Ampere and Volt), and connect it to the battery;
- Set the voltage to a level from 13.9 to 14.3 Volts, and the current to a level from 0.8 to 1 Ampere and turn it on, then leave the battery in this state for 8-9 hours;
- Having completed the steps described above, you will notice that the density of the electrolyte in the battery has not changed, but the voltage has increased to approximately 10 Volts - this is what is required;
- Disconnect the charger from the battery and leave it in this state for 24 hours;
- Next, reconnect the charging station to the battery, but this time set the current to 2-2.5 Amperes. Again we leave the battery to charge for 8-9 hours;
- After this, you will notice that its voltage has increased to a level of about 12.8 Volts, and the density has increased to values of 1.11-1.13 g/cm 3 ;
- As you can see, the desulfation process has begun. To continue it, you need to apply a small discharge current to the battery. To do this, it is best to use a car's high beam lamp, or something similar in load. Leave the battery with the load connected for 8-9 hours. It is advisable to constantly monitor the result so that the voltage does not drop below 9 Volts, while the density remains at the same level;
- After this, we charge the battery again for 8 hours using a charging station with a current of about 0.8-1 Ampere. Then again we leave it standing without a connected charger and load for 24 hours, and then we charge it with a current of 2-2.5 Amps to again increase the battery voltage to a level of 12.7-12.8 Volts. After the second cycle, you will notice that the density has increased to 1.15-1.17 g/cm 3 . Next we load the battery again.
Similar procedures should be carried out until the battery density approaches the ideal - 1.27 g/cm 3 . Such work will allow you to clean the battery plates by 80-90%. Please note that depending on the complexity of the situation, work may take up to two weeks.
Read also: Size of cutting discs for metal
Reasons for education
Deep battery discharge.
The plates of any lead-acid battery are subject to normal sulfation. During operation, light-colored crystalline growths are formed and the more of them, the lower the capacity. Their destruction occurs when the battery is charged. The following factors can disrupt the correct cycle:
- Improper, long-term storage of the battery without charging;
- High temperatures;
- Low temperatures;
- Deep battery discharge (low voltage);
- High acid concentration.
High current charging
During the process of replenishing the charge with a current exceeding 10% of the capacity in absolute value, fine-crystalline lead sulfate does not have time to completely transform into the original active components. Partial sediment remains, and the car owner sees that charging is complete:
- Active gas evolution is observed.
- The electrolyte density returned to normal levels of 1.25–1.31 g/cm3.
- The voltage does not change.
Of course, a one-time situation will not turn out to be critical. But if charging with a high current is repeated constantly, the amount of lead sulfate will accumulate, the structure of the crystals will change, and the battery will begin to lose capacity.
Deep discharge
Deep discharge is the threshold capacity of the battery, after which it has nowhere to discharge. In this situation, almost all of the sulfuric acid ends up on the plates in the form of salts. In order to get it back, you need to start charging the battery as quickly as possible. In theory, everything looks simple: unload, charge, move on. In practice, it is not possible to completely return crystalline lead to its original state. Some of the sediment remains on the plates; subsequent deep discharge only worsens the situation. Each deep discharge reduces the battery capacity by 2–3%. Just 10 discharges will reduce the battery's capabilities by 20–30%, and such a battery will no longer start the engine.
Long parking
If the car is not used, the battery charge may drop to critical. To prevent this from happening, you need to start the engine at least once a month.
Electrolyte problems
If the level is below the mark, some of the plates may become exposed. In this case, active interaction of sponge lead with oxygen occurs, which will further accelerate sulfation. If the level drops, but the solution still covers the plates, the density of the electrolyte begins to increase (water evaporates, acid remains). Increased H2SO4 content also accelerates sedimentation.
The battery is constantly in an undercharged state
In this case, the finely crystalline sulfate turns into large crystals and later into a crust, which is not reduced when charged.
We recommend: Car insurance
Sudden changes in temperature
Lead sulfate is an insoluble compound, but it still has a slight solubility, and it differs markedly in hot and cold solutions. In winter, PbSO4, dissolved in a hot battery while the engine is running, is deposited on the plates after stopping and cooling. With repeated heating-cooling cycles, the mass of the sediment increases. As a result, fine-crystalline sulfate turns into coarse-crystalline sulfate, reducing the battery capacity.
Other methods for desulfating plates
Plates can be desulfated not only using charging stations; there are other methods that are much less effective and less safe. You can resort to them in extreme cases, so knowing them is simply useful:
- Mechanical cleaning of plates. An extremely complex method that requires special skills. It consists of cutting the battery case and removing the plates from it. After this, the packages with the plates are disassembled, and the accumulated white deposits are removed from them. As a result of this, in theory, the working plane of the battery increases. Next, all that remains is to put the battery back together, fill in the electrolyte and charge the battery;
- Use sodium sulfate elimination solution. Almost any chemical compound undergoes dissolution, and sodium sulfate is no exception. Accordingly, to get rid of sodium sulfate stuck to the plates, you can use a special solution that will dissolve it. Trilon B is suitable as such a solution.
Both methods discussed above can lead to permanent damage to the battery. If you want to carry out high-quality desulfation of the battery plates, you should resort to the method of charging and discharging the battery using chargers.
A fully charged car battery refuses to start the engine? For most motorists, this may be a reason to purchase a new battery. However, the problem may not be a serious breakdown, but sulfation.
Covering the plates with a coating of lead salts and thus reducing its capacity does not make it possible to extract sufficient current for ignition. “Treatment” of the unit is carried out using desulfation - loosening the plaque on the gratings and returning lead atoms to the plates. This process can be carried out using several techniques: chemical, mechanical, electrochemical.
Sulfation - what is it?
The operating principle of the battery is based on the energy of the chemical interaction of lead and acid. The lead grid acts as electrodes. Concentrated sulfuric acid is poured in as an electrolyte, which at the first moment forms salts with calcium or lead and envelops the working surface of the grate with a thin film of this substance.
In essence, sulfation of battery plates is the process of deposition of lead sulfate salts on the electrode plates
During normal battery operation, this is a natural process where the electrolyte transfers charge to the plate as a result of a chemical reaction to form metal salts. On one of the electrodes, small damage forms at the site of atoms “torn out” from the surface, and on the other, salts of the element accumulate.
The desulfation process allows you to break up the salt compounds and return the electrolyte composition to its original form, and the lost metal atoms are returned to the electrode.
Desulfation is the removal of sulfuric acid salts from the battery plates
It should be understood that it will not be possible to completely return all the formed compounds to their original form. With proper care and timely charging, such batteries will last for several more years, but at the same time the electrodes become loose and dotted with salt crystals, which no longer break down during desulfation.
Brief video description of the sulfation process:
A decrease in charge occurs due to a large accumulation of crystallized calcium or lead salts on the electrodes, which prevents the penetration of the electrolyte plate to the surface. A lower concentration of charged ions in the electrolyte leads to a decrease in the battery capacity to a critical level, which does not allow the car to receive the charge required for ignition.
This condition of the battery should be dealt with in several ways: chemical, mechanical, electrochemical. All of them have varying degrees of efficiency and are selected depending on the type of battery, state of wear, and other parameters.
The concept of sulfation and desulfation
First, you need to understand the concept of sulfation of battery plates, which will allow you to understand what the reverse process is, that is, desulfation.
Sulfation is the process by which a coating of lead sulfate forms on plates made of lead. It's lead sulfate.
The formation of this substance occurs during battery discharge and is caused by a chemical reaction occurring under these conditions.
Lead sulfate also appears during normal operation. But its concentration and quantity are insignificant. When charging, the components dissolve again without causing serious damage to the battery.
When the discharge is strong, chemical processes occur very actively, which is detrimental to lead plates. Sulfate-coated plates stop taking part in chemical reactions and cannot generate energy. Because of this, the battery capacity decreases, and the battery completely fails over time.
There are characteristic signs that confirm that severe sulfation of the internal plates of the battery is occurring. This is a rapid discharge, the inability of the battery to hold a charge, etc.
Among the main reasons are the following:
- influence of deep discharge;
- exposure to low temperatures;
- elevated temperatures;
- adding electrolyte or acid to the battery;
- long-term storage in a discharge state.
Accordingly, desulfation is the reverse process, that is, cleaning the plates from the resulting deposit of lead sulfate.
Several solutions can be used to reverse the process.
Main features
The most obvious sign that the battery is not delivering the required current due to sulfation is the formation of a gray solid coating on the plates. It is not always possible to consider it due to the characteristics of the battery. For serviceable batteries that are equipped with a removable cover, it is possible to open the device and look into it.
In another version of the battery, if it is completely sealed, such an operation requires cutting the battery, which is unsafe for humans.
Signs of battery sulfation:
- A fully charged battery is unable to start a vehicle's engine.
- Battery capacity has decreased
- Electrolyte density indicators indicate a decrease in the nominal value
- Device cans boil quickly during charging
- The battery charges or discharges unnaturally quickly
To increase the service life of the battery and return to working condition, it is necessary to properly desulfate the device.
How to prevent capacity loss
From the above it is clear that in order to avoid sulfation and keep the battery in working condition for as long as possible, it must be constantly kept in a fully charged state. If it's a little discharged, there's nothing to worry about, but it needs to be charged as quickly as possible. Long-term storage of even a slightly discharged battery can lead to sulfation.
The problem is that all batteries tend to self-discharge. After just a couple of days, a fully charged battery becomes slightly discharged. So the batteries need to be constantly recharged. If the battery is installed in the car, you drive it almost every day, and the car's electrical system is working properly, then the battery will recharge and you have nothing to worry about. But if the battery is simply stored, or you drive the car once a week, then the battery needs to be recharged regularly.
How to eliminate plate sulfation
Desulfation refers to the effect on electrodes and plates in various ways that help eliminate the resulting deposits of calcium or lead salts. There are different types of cleaning: mechanical, chemical or using inorganic additives, electrochemical using a charger.
The simplest and fastest method of desulfation is considered to be mechanical cleaning of the plates from the formed salt crystals. Older or serviced batteries allow you to remove the cover and gain access to the plates and electrodes.
These components are removed from the battery manually and cleaned in the same way - the plaque is simply scraped off from the surface and crevices until completely eliminated as far as possible. Modern units are often produced as maintenance-free models. This makes it impossible to get to the jars with electrodes to remove and clean them.
To clean the plates of a dead battery using this method, you need to perform a number of operations:
- Remove or cut off the upper part of the housing for batteries to be serviced.
- Clean each of the plates by hand , carefully so as not to damage the structure of the electrodes;
- Place the cleaned plates in their place in the containers, maintaining the required gap between each;
- Make the case airtight , solder the removed lid;
- Fill the jars with electrolyte of the required density;
- Check the performance of the battery, “adjust” the density of the liquid to the same level in all banks, not allowing a difference of more than 0.01 kg/cubic meter. cm and the electrolyte concentration is not lower than 1.25, but not higher than 1.31 kg/cu. cm.
This method is not applicable for EFB batteries, since each group of electrodes is separately sealed in a separator designed to prevent the plates from shedding.
In this design, the density of the electrolyte in the jar and the package itself (separator) differs, which will damage the device if the integrity is damaged. This factor prevents mechanical desulfation.
Chemical additives
The essence of the process is the introduction into the cavity of the jars with electrolyte of special additives with a chemical composition that affects calcium or lead sulfates. During charging, solutions with additives slow down the formation of salt deposits on the electrodes, which returns the battery to almost nominal charge.
Most often they choose Trilon-B , however, this solution does not work equally effectively on all batteries. The reaction depends on the design features of the battery, model and technical parameters. There is a 50/50 chance that the chemical desulfation method will work.
Important! In many batteries, manufacturers coat the plates with a paste containing lead oxides to increase battery performance and service life. When using additives, such a layer quickly dissolves and chemical “reanimation” of the device leads to its death.
The composition of "Trilon-B" includes 5% ammonia, 2% acid organic derivative of sodium salt, distillate. These components are inert to lead, but react well with deposits on the electrodes. In industry, such a solution is used to convert insoluble salts into soluble ones.
Procedure for chemical desulfation:
- In accordance with the above proportions, a Trilon-B solution is prepared
- The battery is fully charged
- The battery cans are washed with distillate 2-3 times.
- The solution must spend at least an hour in the cavity of the cans so that the chemical reactions are completed and gases stop being released.
- The inactive solution is drained upon completion of the reactions (pumped out without turning the device over)
- Rinse the inside of the jars 1-2 times with distilled water.
- New electrolyte, density 1.25-1.27 kg/cubic. cm, is poured into each jar, its density is checked and adjusted to one value with a spacing of no more than 0.01 kg/cu. cm for each container
- The battery is fully charged, the fluid concentration is adjusted
Read also: How to disassemble a screwdriver motor
Electrochemical method
The most productive method of desulfation is considered to be electrochemical, which is carried out with a special charger.
The essence of electrical desulfation is to pass a current through the electrolyte with higher rates than the nominal values of the battery. This leads to natural dissolution of accumulations of lead or calcium salts in the liquid surrounding the plate and dissolution in it, increasing the density of the electrolyte. This brings the battery performance back to normal.
Restoration with a simple charger, do it yourself
You can desulfate the battery yourself using a special or standard charger.
A conventional charger can be automatic with the ability to regulate the current and voltage supplied to the terminals and the “Desulfation” mode, or simplified with the need to control the process. The most convenient option is an automatic pulse charger with a desulfation mode.
Charging steps with an automatic charger with desulfation mode include the following steps:
- The negative and positive terminals of the automatic device are connected to the corresponding poles of the battery;
- The required voltage and current strength are adjusted, and the “Desulfation” mode is turned on;
- Equipment is connected to the network;
- The battery begins to charge, the plate renewal process occurs at the negative terminal;
- At the end of the charging process, until its capacity and electrolyte density are completely restored, the power supply is disconnected and the battery terminals of the automatic device are removed.
Process time depends on many factors:
- Degree of battery discharge;
- Equipment containers;
- Level of sulfation of electrodes.
To calculate the average charging time, divide the battery capacity by the average charging current. Most often, it takes from 15 hours to 3 days to completely restore the equipment.
Instructions for charging the battery with a conventional charger
For this type of battery charging by electrochemical method, it is necessary to regularly monitor the process and constantly intervene in it. For reliability and accuracy of charging, the instructions are developed for a battery with an electrolyte density of 1.07 g/cubic meter. cm and a voltage of 8 V at the equipment terminals. Without receiving voltage, this device begins to boil after 15 minutes during typical charging.
To desulfate, do the following:
- Provide a room with good air circulation for charging the device;
- Check the level of electrolyte in the battery jars and replenish it, if necessary, with distilled water;
Important! It is prohibited to dilute with concentrate or electrolyte of any density before charging!
- Connect the battery to the charger;
- Set the current to 0.8-1 A and voltage 13.9-14.3 V for about 8-9 hours. These manipulations will allow you to increase the voltage at the battery terminals to 10 V, leaving the electrolyte density level unchanged;
- Disconnect the battery from the charger and keep it in this state for about a day;
- The battery is reconnected to the charger with new current parameters: power 2-2.5 A and voltage 13.9-14.3 V for 8-9 hours;
- After recharging, the battery parameters will change: the electrolyte density will increase to 1.12 g/cc. cm, and the voltage at the terminals will rise to 12.8 V;
- This indicates the beginning of desulfation. For the next step, you need to discharge the battery to 9 V by connecting to the terminals of an active resistance - a lamp or headlight. The average time for a discharge is 8-9 hours. The density of the electrolytic liquid will remain at 1.12 g/cc. cm;
It is necessary to control the battery discharge process, since the final voltage must remain at least 9 V.
A subsequent pair of charging and discharging the battery according to the above scenario will increase the electrolyte level to 1.16 g/cc. cm. It is necessary to repeat the cycle until the density reaches 1.26 g/cubic meter. cm or will not come close to the nominal 1.27 g/cc. cm.
Important! The duration of battery desulfation work using a conventional charger, depending on the condition of the battery and the complexity of the process, can take up to 2 weeks.
As practice shows, such manipulations renew the battery by 80-90%.
Video
You may find it helpful to watch the video provided on battery desulfation.
Homemade products from a washing machine engine:
1. How to connect a motor from an old washing machine through a capacitor or without it 2. Homemade emery from a washing machine engine 3. Homemade generator from a washing machine engine 4. Connecting and adjusting the speed of a commutator motor from an automatic washing machine 5. Potter's wheel from a washing machine machines 6. Lathe from an automatic washing machine 7. Wood splitter with an engine from a washing machine 8. Homemade concrete mixer
Desulfator or do-it-yourself dedivan charging
Our task is to obtain a long-lasting chemical battery from a battery. (c) dedivan
Where to begin? I'll start with trance. Everything in order - Take a ferrite ring K28x15x9. This is the most popular size. I warn you right away - Chinese yellow rings will not work from the power supply - they are not ferrite. The permeability can be from 600 to 3000. This is because we will not drive it through a full magnetization loop, to save losses in the core. Therefore, he has a reserve. First of all, we make a gap. A 0.4 mm thick diamond cutting wheel produces a gap of about 0.5 mm. Well, it’s like someone’s hands are shaking.
Secondly, we wind the windings. The first thing is isolation, for my grandfather this is sacred - never dangle on a bare ring. The varnish on the wire will be scratched, the voltage on our turns is up to 500 volts, it will break out sometime, usually at the most crucial moment. We take a 0.8 wire - we calculate that 60 turns should be removed along the inner circumference, turn to turn. We start to shake - here’s where my grandfather’s fingers cramp - there’s no longer the tension that was before. Only 56 were removed. But the trance has a reserve. And then the secondary winding - there should be 10 times fewer turns, only 6, but we wind it in several wires. It’s easier to wind this way - the wire is softer than one thick one, and the connection between the winding and the core is better. We also select the wire based on the condition of filling the inner circumference of the ring. Turn to turn in one layer. I got 4. Then we solder them in parallel on the board.
Well, now... let's connect this trans to the circuit. Our key is a field-effect transistor for a current of more than one ampere and a voltage of more than 400 volts. We apply a pulse of 50 μs more than +5 volts to the input. During this time, the current in the primary increases to approximately 1 ampere. When the key is opened, the energy of the magnetic field seeks an outlet - and finds it through the secondary winding and diode into the battery. The voltage in the secondary jumps to 20 volts. But according to all transformer rules, the current in the secondary is 10 times greater than in the primary. At the same time, it is clear that in the primary there will be 200V, and taking into account emissions from parasitic inductances, up to 400. That is why the field switch should be installed like IRF 740,840, etc. Well, don’t touch it with your hands - inductance is simple - it doesn’t care what your body resistance is - the current will always provide 1 ampere. So the comb may fly off. There are practically no diagrams - just installation rules. The power and ground wires are separated because a strong short pulse runs in the secondary wires and even on a few centimeters of straight wire a large emf occurs. The battery also shows a surge in voltage - up to 5 volts, depending on how bad the battery is. Therefore, we also install filters everywhere, both to power the circuit and the load.
The circuit works like this: we accumulate energy for 50 microseconds, then we give it back to the battery for 5 microseconds, and we wait 500 microseconds for the battery to digest it so that it can be absorbed. You can send impulses less often. In a practical scheme, this is exactly what needs to be regulated. If the voltage on the battery increases, and we do not have time to consume all the energy, then we need to slow down.
This is a simple pulse generator for pumping. It gives a 50 µs pulse every 500 µs.
50 μs is a plus, after which there is a pause of 500 μs. 50 μs - the key is open - we are saving energy. At this time, on the secondary side there is a minus - nothing goes into the account. And only after the switch is closed, an emf pulse occurs. 5 microseconds - we give it back. And 500 microseconds - we wait for digestion. Well, or 495, to be precise.
How to make your own desulfator
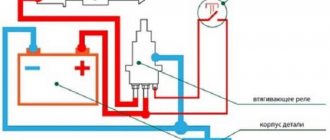
A desulfator is a device capable of autonomously cleaning the battery without the need to remove it from the vehicle.
The process will require removing at least one terminal connecting the battery to the car. This is done in order to protect the machine’s electronics from possible loads. In addition to cleaning the electrodes from salt deposits using a desulfator, you can perform regular maintenance on the working battery, which can significantly extend its service life.
The operating principle of the equipment is based on receiving power from the battery and generating high-frequency short-term pulses in this circuit. When resonance occurs in lead atoms and lead salt molecules, reverse sulfation of the plates is initiated. This process restores the resistance and capacity of the battery to its original values.
The main disadvantages of the “miracle equipment” are the long desulfation period - in rare cases it reaches a month or at least a day, as well as the inability to restore approximately 10% - 15% of the batteries with it.
A simple low-power desulfator circuit is popularly called a blinker. Most often, such a device can effectively help restore the battery.
To make it you will need:
- Turning relays , imported ones with a voltage of 12V and a power of 21 W are better suited. To increase the working time, it is worth replacing the capacitor in the device with an analogue of a larger capacity. Suitable for 100 uF for relay operation for 3-4 s
- The relay is 5-pin with normally closed contacts (contacts 3 and 4 are closed, contacts 1 and 2 are control). Instead of an imported one, a domestic relay from a Soviet VAZ is suitable
- Load resistors or light bulbs
- Soldering iron and connecting wires
A basic diagram is drawn up with the main points:
- The negative terminal of the battery is connected to the output of the same charge of the device;
- A rotary and 5-channel relay is connected to the “-” output on the battery with the corresponding charge outputs;
- The output of a 5-channel relay of a similar charge is connected to the charging equipment at “+”;
- The turn relay and the 5-channel relay are connected to each other, as well as the output of both relays with the “+” terminal of the battery;
- The turn relay is loaded with a light bulb or an active resistor;
- It is advisable to monitor the assembly and check the functionality of the device by connecting an ammeter and voltmeter to the circuit between the device and the battery.
A textolite plate is used for the base for fastening all elements. There is a possibility of the rotary relay breaking due to the closed state of outputs 3 and 4. This will prevent the battery from being discharged.