History of nickel silver
The appearance of nickel silver is directly related to cupronickel, which was invented in China. The Chinese protected the cupronickel formula no worse than the secret of creating silk. This is not surprising, because it had completely unique qualities, could replace silver, but at the same time was several times cheaper.
Over time, cupronickel began to be popular among European secular society, which is why they really wanted to reveal the formula of this strange alloy. The best minds in Europe tried to solve this riddle. Whether long or short, chemists discovered the composition of the alloy, which they thought included copper, nickel and zinc. However, they were unable to calculate the ratio of these metals.
I don’t know how things went there, but apparently they didn’t pat the chemists on the back and suspended all research for almost 300 years. And in the 19th century, German researchers were still able to produce a sample of “cupronickel,” which in fact turned out to be not cupronickel at all. Research has shown that this metal was much stronger and more stable; the material is destroyed only in boiling hydrochloric and sulfuric acids.
Thus, German chemists managed not only to repeat the Chinese formula, but also to discover a new alloy of nickel silver. Silver, cupronickel and nickel silver look exactly the same, at first glance it is impossible to distinguish them. However, the cost of nickel silver is the lowest. It is precisely because of its similarity with silver that jewelers began to actively use nickel silver.
We are also preparing to cast our products from nickel silver. This is economically beneficial for you, our dear customers, because its cost will be in the same price category as bronze. This material is resistant to corrosion and temperatures, does not oxidize, does not turn black and does not change its properties over time. Jewelry made from nickel silver looks as much like silver as possible.
It is possible to distinguish them only by checking the sample. So, silver will have a 925 standard, but nickel silver will not have it at all, or in some cases they will put three letters “mnc”, which means copper, nickel and zinc.
Cost of spoons with a stamp
The table set is a good gift. But it also needs to be chosen correctly. If it has a famous manufacturer, it is valued much higher. The cost will increase if there are special linings and gift wrapping.
An example of the USSR stamp on a spoon.
Here's what you need to pay attention to when purchasing:
- name and status of the manufacturing company;
- year of creation;
- packaging;
- external characteristics.
If the cost is significantly low, then the product is probably counterfeit. The most expensive and rare devices can now only be bought from collectors. Expensive sets always have an additional manufacturer's mark.
How to clean nickel silver?
There are a huge number of options available and you can clean nickel silver at home. To do this, you can use toothpowder or toothpaste, dishwashing powder, salt or soda. These are simple solutions that will help bring back the “newness” of your jewelry. Very soon you will be able to purchase nickel silver products in our store. In the meantime, don't forget that you can always buy silver or bronze. Products from which are not inferior in quality and characteristics.
Silver is the favorite metal of jewelers
Silver has been known to man since the 5th century. BC, but “younger” than gold. Prehistoric Sardinia is considered the birthplace of silver. In Assyria and Babylon, silver was a symbol of the Moon and was considered sacred. In the Middle Ages it was popular among alchemists.
This is a precious metal with a white, shiny color. Occurs in nature in the form of ore or native state. Silver is harder than gold and softer than copper. Very ductile and easily rolled into thin sheets and wire. It cuts and polishes well. Silver does not oxidize in air or when heated. But it darkens under the influence of sulfur or hydrogen sulfide, which are present in the air and the human body. The higher the silver standard, the less oxidation .
In humid climates or indoors, metal darkens faster.
Features of silver
A special property of silver is its sonority. The purity of the ringing of church bells and instrument strings is achieved by adding silver. The thinnest layer of silver is applied to the surface of the mirrors. This is a material for jewelry, dishes and cutlery. For filigree production and enamel products. Silver can also be contained in gold and platinum
alloys, making them lighter. Used for soldering copper and brass products.
Silver is not affected by boiling caustic alkalis. It dissolves in nitric and hot sulfuric acid.
Nickel silver casting
Friends, as it turned out in practice, casting nickel silver is not a simple matter. When I tried to independently form a nickel silver alloy using formulas from the Internet, I was not successful. The defect rate reaches 90%, which is very high.
Consultations with specialists who have experience working with nickel silver alloy showed that a good indicator of the quality of injection molding in open systems is a defect rate of 50%. As a result, by selecting the metal recipe, we managed to reach the quality/defect mark of 50/50.
Later, it became known for certain that obtaining 100% casting quality is possible only by using certain alloys and only one type of injection molding machines. Although this fact can also be considered dubious.
Nevertheless, in the end we achieved almost 100% quality of finished products. The actual defect rate at the time of writing this article is 95%. Out of 100 decorations placed on the Christmas tree, only 5 were defective. Upon closer examination, it turns out that 5 defective works can be attributed to defective wax models, bubbles under the surface of the wax.
Now the nickel silver alloy is in mass production and in principle no problems arise with its casting.
When casting this metal, special attention must be paid to the casting machine and not to the alloy itself. It is also necessary to strictly adhere to the production technology as a whole, from the moment of forming the tree, the time between evacuation of the flask and before placing it in the muffle furnace.
The diameter of the gating system is also important, although on certain closed-type machines this parameter is not important.
Tin is the purest metal
Tin has been known to man since the 4th millennium BC. Coins were minted from it and vessels were made. In ancient Rus', churches, doors and iconostases were decorated with openwork tin casting.
Tin is silvery-white in color, but darker than silver. This is the purest metal and does not rust. Tin utensils are highly valued - they do not in any way affect the taste and smell of food. Soft, tough metal. Does not oxidize in air and very slowly in water.
Tin Plague
Tin is resistant to corrosion, but when strongly cooled, it loses its metallic properties. Individual gray spots appear on the product. With further cooling they spread over the entire surface. The transformation of tin into powder is called the “tin plague.” «
. You can stop it by heating it to +18 °C.
When exposed to air, tin fades beautifully. This velvet shade is highly prized by collectors. Tin is not dishwasher safe and is best polished. Utensils for eating must be marked 95%. But it is worth considering that tin melts at a temperature of 170-230 °C.
Applications of tin
Currently, pure tin is not used. It is used for alloys with copper and lead. Also obtained from tin «
gold leaf
"
in the form of thin leaves or powder. This is tin disulfide, a shiny mass. Durable and gold-like in color. Retains shine on products indoors and outdoors for a long time. (A. V. Flerov “Materials science and technology of artistic processing of metals.”)
Pewter began to be used in the Middle East. Items from it were discovered in an Egyptian tomb from 1450 BC.
Cupronickel alloys
Cupronickel alloys have different percentage compositions. The most common cupronickel alloy contains from 5 to 30 percent nickel, with the remaining percentages being iron (0.8 percent) and manganese (0.1 percent). There are other alloys that are very similar in their chemical composition to cupronickel, but have different names:
- Cupronickel, similar to cupronickel, contains only copper and nickel in equal proportions;
- Monel, contains up to 67 percent nickel;
- nickel silver, contains additional zinc;
- Constantan, very close in chemical composition to cupronickel, contains 55 percent copper and 45 percent nickel.
Copper is the first metal of mankind
Copper —
the first metal for the production of tools after stone. According to Pliny, “Copper was first mined in Cyprus.” Hence its Latin name.
Pinkish-golden, red-brown soft metal. Viscous and viscous. Changes shape under impact and is very flexible. It is easy to give it any shape and relief. You can bend the most complex and bizarre ornaments and roll sheets up to 00.5 mm. Filigree and embossed material. Easily soldered, silvered and gold plated. Enamel adheres well to red copper. Pure copper is not attracted to a magnet.
When exposed to air, copper oxidizes and turns green or black. This film of oxides protects the metal from further corrosion. Therefore, products made from pure copper are well preserved without special coatings. Pure or red copper is not rarely used, but is more often used for alloys. And also for the production of red colored glass, enamel and smalt.
Bronze. The most ancient alloy
Bronze is an alloy of copper, zinc and tin. Ancient people learned to smelt bronze from copper and tin ore. This period replaced the Copper Age in human history. Ancient sculptors cast bronze statues as early as 470 BC. The most ancient bronzes contain 88% copper and 12% tin. But later they began to add zinc, improving the properties of the metal. The color of bronze varies from red to yellow (90-85% copper) and from white to steel gray (50-35% copper).
Properties of cupronickel
Cupronickel is very similar in appearance to silver. Sometimes it can be very difficult to distinguish cupronickel silver items from silver items. Cupronickel alloy is a well-known imitation of silver. Especially the cupronickel alloy perfectly imitates silver in jewelry. Some unscrupulous traders sometimes try to sell nickel silver items by passing them off as silver.
Cupronickel silver products are often coated with silver. Cupronickel silver-plated cutlery and crockery look like real silverware. Therefore, when buying silver jewelry and cutlery, we need to look very carefully at what we are buying. Cupronickel has greater mechanical strength compared to silver. Cupronickel alloy has high corrosion resistance and good plastic properties. Due to its high plastic properties, cupronickel alloy is easy to process. Cupronickel alloy produces elegant and sophisticated products.
Where is it used?
The properties of cupronickel ensured its use by industrialists, decorators, and bankers.
Industry, medicine
The structure of the alloy allows all types of mechanical processing - cutting, forging, stamping, soldering. And at any temperature.
This determines the areas of application of cupronickel:
- High precision resistors, heat generators.
- Production of components for trawlers, cruise ships, ocean liners. And private yachts of the premium segment.
- In combination with lithium, nickel-iron alloys are purchased for the production of batteries. They are three times more effective than their analogues. Lithium, as the lightest metal, reduces the massiveness of the filling of nickel-zinc or ferrous batteries.
Cupronickel is a material for medical instruments: scalpels, forceps, etc.
Plus the possibility of decorative processing of products (chasing, cladding, polishing).
Decor
Decorators work with alloy in two directions:
- Jewelry. Rings, earrings, necklaces, and cufflinks made of cupronickel are indistinguishable from silver in appearance. However, large companies are not interested in the alloy due to its low cost. It is more often done by hand-made masters. The processing makes them look like museum pieces.
- Dishes. Sets of cupronickel tableware - spoons / forks / knives / sockets / tureens - are a pride and heirloom in many homes. Their advantages: they are several times cheaper than silver ones, but more durable and look presentable.
Especially good are gold-plated, silver-plated, blackened or other coated surfaces.
Applying such a layer is not only a tribute to aesthetics or status. Pure cupronickel imparts tangible metallic notes to food. Copper is toxic, especially when hot.
Banking sector
Wear resistance, coupled with low cost, prompted the National Banks of many countries to issue cupronickel coins.
Almost all silver-colored coins in circulation are made of cupronickel.
In the USSR and Russia, coinage is controlled by GOZNAK:
- In the 1930-1950s, nickels, 10-, 15-, 20-kopeck coins were issued.
Today, the subject of numismatists' hunt is a Soviet patch of the 1937 model.
- Since the 70s, the line has been supplemented with anniversary and regular “rubles”.
The last Soviet coin was the bimetallic 10-rublevik.
In Russia, until the mid-1990s, coins were minted in denominations of 10, 20, 50, 100 rubles. After the redenomination of 1997 - 1 and 2 rubles.
How to distinguish cupronickel from silver
6 ways to distinguish cupronickel from silver:
- if the product bears the abbreviation MNC (an alloy of copper, nickel and zinc) instead of a sample, it is cupronickel;
- hold the product in water - nothing will happen to the silver, and cupronickel will become covered with a green coating;
- rub the surface of the product with a lapis pencil - the silver will not change, but a dark spot will appear on the cupronickel;
- you can weigh two similar products - nickel silver is lighter than silver;
- smell the object being examined - cupronickel smells like copper;
- use iodine - silver in the sun will darken in the place where iodine was used.
Cupronickel has a melting point of 1170 degrees, higher than that of silver (960 degrees). The melting point of various cupronickel alloys can vary depending on the component composition.
Cupronickel alloy has a structure that allows it to be mechanically processed both hot and cold. Cupronickel is well processed, alloyed with other metals, cut, forged, embossed, stamped, polished and soldered.
3 Ways to Effectively Clean Gold to Make It Shine
Etymology
The alloy of copper and nickel was known back in the 3rd century BC. e. like "white copper". The modern name comes from the names of its “inventors” - the French Maillot and Chorier. French name Maillot-Chorier
in German it was distorted into
Melchior
, and then spread into Russian.
In modern European languages, alloys of copper with nickel are often associated with the Latin name of its components - Cupronickel
(in contrast to the names of alloys of copper with nickel and zinc, which are usually called nickel silver -
Nickel silver
, German silver -
German silver
, new silver -
New silver
,
Neusilber
; the name
Mailechort
is also sometimes used in relation to alloys of copper with nickel and zinc - nickel silver).
Cupronickel silver cutlery: alloy composition
An alloy of light-colored metals, which is now universally known under the name cupronickel, appeared in ancient times. In Mesopotamia, around the third century BC, it was called white copper and was widely used in the production of utensils, various vessels and household items. Then, in numerous wars and under the influence of time, the secret of alloy technology was lost for several thousand years.
It is believed that cupronickel was invented, or rather recreated, by the French inventors Maillot and Chaurier in the nineteenth century. Despite the fact that the invention was made by the French, cupronickel silver was called “German silver” for a long time. Kitchen utensils, dishes, church vessels, and decorations were made from it.
Repair mark
A square divided in half.
1 description option. Near the wooden overlay there is a restoration mark - a rectangle divided in half. Such terminals had been installed for several years since 1928, while revolvers (triggers, springs, sights) worn out in the battles of the imperialist and civil wars were being repaired.
Option 2 description. Small repair marks - a rectangle divided in half (read “overhaul in 1932”, when they began to remake the sighting parts), which were placed since 1932 on revolvers produced earlier, i.e. The revolver with this mark was produced before 1932.
A diamond means a complete overhaul, which in turn means a complete or partial re-sorting of parts of the revolver. During the overhaul, they could change the drum and re-barrel, but this is less likely than simply replacing a worn-out trigger. The diamond sign is less common than a rectangle divided in half. Accordingly, it means a non-standard overhaul, or a special workshop. I think this sign indicates some kind of “fine” work on the revolver.
Melchior - what is it?
It is an alloy of copper and nickel. It belongs to the group of non-ferrous metals. May have minor amounts of iron, silver, cobalt, manganese, zinc and other components. The higher the percentage of nickel, the more the anti-corrosion properties of the alloy increase, and its strength and durability improve.
Cutlery and dishes made of cupronickel:
- They heat up at a lower rate than products made from other metals;
- The alloy is not subject to corrosion, does not oxidize, objects made from it do not rust;
- The material has a good degree of strength, the dishes do not deform, the cutlery does not bend during use;
- Tableware and cutlery are distinguished by their sophistication and beauty;
- Due to its composition, cupronickel has a beneficial effect on the human body;
- The items are relatively inexpensive.
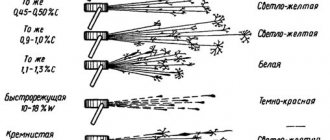
The main color can vary from light tones, with a slight yellowness, to orange.
Technological characteristics
During the melting process, the red tint of the copper is absorbed by the nickel. The result is an alloy of white, white with a blue or greenish tint with a deep glossy texture.
When lead is added to the alloy, the glossy shine fades and a gray tone clearly appears. The specific gravity of the copper-nickel alloy is 7.5 grams per cubic meter. cm.
For further processing, ingots, rods, strips, pipes and wires are produced. The main processing method is cutting and pressure forming. Nickel silver can be heated and cooled, forged, and minted.
When using nickel silver as tableware, the metal is plated with silver or gold. For instruments and electrical products, the metal is polished to a mirror finish.
When covering cutlery, the layer of silver or gold plating is regulated by state standards - for forks and spoons it must be at least 24.00 microns, for knife handles 18.0 microns.
Composition and main characteristics
Cupronickel is an alloy whose composition is 70–80% copper and 30–20% nickel. Contains no more than 1% iron and manganese, 5% cobalt.
It is much stronger than silver and lighter in weight. No magnetic properties. The higher the percentage of manganese and iron in the composition, the greater the ductility, density and thermal conductivity it has. Reacts with other metals, quickly oxidizes under the influence of water and air, so cannot be used in the dishwasher.
There are several types of compositions, which are called cupronickel. The name and technical characteristics depend on the percentage of nickel in the finished material and the presence of other components:
- Nickel silver is an alloy consisting of about 40% copper, up to 15 nickel and up to 45 zinc. Often other metals are added to its basic composition, sometimes it has a bluish or bluish tint. The composition is very durable, strong, elastic.
- Monel is an alloy with a predominance of nickel, it contains up to 67%, copper up to 35%, iron and manganese no more than 2.5%.
- Brass is a material containing up to 80% copper, up to 50% zinc, with nickel, iron, and tin. Previously, this composition belonged to the cupronickel group, so objects made of silver-plated brass were also called cupronickel.
About copper, brass, tin, bronze and other alloys.
Due to my passion for jewelry, I became actively interested in what we make everything from - what kind of metals are they? what properties do they have? What is the gold standard in karats? What kind of turquoise do I buy? What types of fakes are there? etc. I thought that this was interesting not only to me. so I’ll slowly post it all here.
(all information is “pulled” from the Internet from different sites)
Copper is a metal designated in the periodic table of chemical elements as Cu (Cuprum). Copper is one of the first metals that people began to use in ancient times. As a result, today all copper deposits have been selected, and it is mined from low-grade ores.
Man discovered copper before all other metals except gold. Even in prehistoric times, copper was used by Stone Age people.
Copper is found in a fairly pure state - in nuggets and grains of metal without impurities. Perhaps the first time a person picked up these nuggets from the ground was because they were beautiful. Then the man made a great discovery, finding out that these strange reddish stones can be given any shape. This was a simpler method of making weapons and knives than chipping flints.
Much time passed, and other people discovered that they could melt red stones and make cups and jugs from the molten mass. Then people began to mine copper and make all kinds of devices and utensils from it.
For thousands of years, copper remained the only workable metal because gold was not only too rare to be considered, but also too soft for practical purposes. Copper tools may have been used in the construction of the great pyramids of Egypt.
When bronze (an alloy of copper and tin) was discovered, even more copper was mined. But after the discovery of iron, copper began to be used in small quantities, mainly by peoples at a low level of civilization, until the era of electricity arrived. Since copper is a good conductor of electricity, it is widely used in modern industry.
Very few people have seen pure copper and are unlikely to recognize it if they see it. It is a shiny silvery substance with a slight pinkish tint, which turns reddish in color as it comes into contact with air. The copper we typically see is reddish-brown in color. This is the color of copper oxide, which is formed as a result of the interaction of metal with air.
Most of the world's copper exists in combination with other substances from which it must be separated before use. It is often adjacent to sulfurous substances, which can also be combined with iron and arsenic, which makes it difficult to purify copper.
Copper has some other advantages, besides the fact that it has outlived many other metals. It has high strength, but nevertheless is flexible enough that it can be stretched and given any shape through processing. It conducts heat no worse than electricity. Copper can be carved and engraved. But it's not easy to break. It can also be used to create alloys such as bronze and brass by combining it with other metals.
Brass (yellow copper) is one of the most useful and most commonly used alloys. Its composition varies over a fairly wide range depending on its purpose, but the main components - copper and zinc - are usually found in a ratio of about 2 parts copper and 1 part zinc. (Although zinc was discovered in the 16th century, brass was already known to the ancient Romans and was prepared by them by reducing the smelting of copper (or oxygen copper ores) with galmay, which was believed to have the property of turning copper yellow.This method of preparing brass was also practiced in the Middle Ages and survived until our century, but is now completely left). Brass sometimes contains trace amounts of tin and lead. Brass is harder than copper and therefore more difficult to wear; it is very malleable and viscous and therefore easily rolled into thin sheets, flattened under the blow of a hammer, drawn into wire or stamped into a wide variety of shapes; it melts and casts relatively easily at temperatures below the melting point of copper. Although the surface of brass, if not varnished, turns black when exposed to air, yet as a mass it is more resistant to the action of the atmosphere than copper. Finally, it has a beautiful yellow color and polishes well.
Many people do not wear brass jewelry because it can cause skin irritation and allergies. This occurs when nickel is added to brass. Yes, brass with the addition of nickel has a beautiful tint, it looks richer and more expensive, but it is these expensive brass jewelry that cause the most severe skin irritation. Tip: Buy cheap brass or look for the word Nickel free on the label.
How to determine the authenticity of cupronickel at home?
It is very difficult for an inexperienced person to distinguish the alloy. There are tests that can determine the alloy:
- Silver-plated or gold-plated devices are marked - MN, MNTs (copper, nickel, zinc). Precious metal items only have a digital hallmark.
- Drop clean water onto the item being tested. After 2-3 hours, the silver will remain unchanged, and a green coating will form on the cupronickel.
- Silver is heavier than cupronickel, so items of equal size will have different weights.
- Having rubbed cupronickel, you can smell copper, silver does not smell of anything.
- If there is no mark on the product, most likely it is cupronickel, even coated with valuable metal.
Markings on spoons
Modern industry does not produce devices from precious metals for mass consumption due to their fragile structure and high cost. To improve the quality and increase the service life of products, various metal alloys are used, giving an external resemblance to gold or silver.
High-quality tableware is indicated by markings, thanks to which you can determine the alloy material used to make the product. Domestic manufacturers, when minting a mark on a spoon, use the following designations in marking:
- MELCH (MN) – cupronickel alloy;
- MNC – analogue of cupronickel – nickel silver;
- AL – aluminum;
- STAINLESS – made of stainless steel.
Some devices are covered with a thin layer of precious metal on top. In this case, a double mark is applied, which includes an indication of the sample number. Silver spoons are marked using the numbers 925.
Many buyers confuse nickel silver devices with nickel silver alloy, which is explained by the high degree of external similarity of the materials. Their difference is that by adding manganese, a cupronickel silver product is obtained, and nickel silver is an alloy that includes zinc. The basic basis of both materials is the same - it is copper with nickel. Having studied the markings, the buyer will be able to find out the composition of the MNC alloy on the spoon, what it is, and how much such spoons cost.
How to make cupronickel dark?
Darkening nickel silver, nickel silver, brass, and other alloys containing copper makes objects more attractive. Under normal conditions, the process can last for years, but with the help of chemical solutions, artificial aging can be achieved instantly.
Before patination, you need to check safety precautions, since some chemical solutions are extremely toxic and emit fumes that are harmful to health. It is necessary to provide exhaust and fresh air supply.
The products must first be cleaned, degreased and washed with warm water, and dried thoroughly.
There are several ways to darken alloys:
- Using “sulfur liver” - mix powdered sulfur with potash 1:2, then put the composition in an unnecessary tin on the stove and after melting begins, heat for another 15 minutes. Cool, grind into powder, store in a sealed container. To darken the object, immerse it in a solution of 1 liter of water and a mixture - from 1 to 20 g. The more powder, the darker the product will become.
- Nickel silver alloys can be darkened with copper sulfate dissolved in water, into which ammonia is poured until a rich blue color is obtained. The object is immersed for some time in the resulting liquid and then slightly heated.
- You can treat the item with a solution of platinum chloride or immerse the item in it. If the darkening is insignificant, then pour a little hydrochloric acid into the solution.
How to use and care
Cupronickel, like fine silver, fades and darkens over time. Even with proper storage, zero humidity in the room is unattainable.
Cleaning
The easiest way to add shine is to buy a special cleaner for cupronickel. Its disadvantage is the need to thoroughly rinse the item after cleaning. Especially dishes.
Generations of owners have tested methods of caring for nickel silver using improvised means:
- Apply a mixture of moistened soda to flannel, suede or other soft fabric. Rub the surface until results are obtained.
- Pour ammonia over the products, then rinse with running water and wipe dry. You can work wearing a mask.
- Boil cupronickel in a decoction of garlic, onion peels or crushed eggshells.
Cleaning with tooth powder, toothpaste, crushed chalk, or products containing bleach is excluded. The first three will scratch the product. The latter will make the cupronickel darken.
Usage
After washing cupronickel dishes after a meal, rinse them with a soda solution (45-50 g per liter of water).
Dishes or other items are immediately wiped dry to prevent them from becoming dull.
Regular thorough cleaning of jewelry or devices will not hurt even if they are used constantly. Resuscitating “neglected” cupronickel will take more time, effort and nerves.
It is better to store products (dishes and jewelry) closed in their “original” packaging. Alternatively, wrapped in cling film or foil. This will minimize mechanical or “wet” effects. That is, they will not be scratched or darken.
Hallmark on nickel silver and cupronickel: what are the differences?
Often buyers want to purchase elegant and high-quality products known since the times of the USSR. But many do not suspect that genuine cupronickel dishes and cutlery have stopped being made since the collapse of the Soviet Union, in the middle of the last century. No less beautiful and durable objects began to be made from nickel silver alloy. It is also called cupronickel, but it contains zinc.
Nickel silver devices are almost impossible to distinguish from silver in appearance. Only that upon contact with human skin or air, silver oxidizes and acquires a dark color, but nickel silver products remain unchanged.
To distinguish cupronickel from nickel silver, you need to look at the mark - cupronickel has the sign MN (copper, nickel), nickel silver is marked with the letters MNTs (copper, nickel, zinc). If there is no mark on the item, then you need to find out the approximate date of manufacture or carefully consider the color of the item. Unlike nickel silver, cupronickel will have a yellowish tint.
Mnc on a spoon - what is it, and how much do such spoons cost, their features
Many people have old cutlery at home, sometimes you can see interesting markings on them. Not everyone knows the MNC on a spoon - what it is, and how much such spoons and forks cost. Previously, such things were very common. Each letter corresponds to one of the elements of the metal alloy that was used to make the product.
Purpose of markings on spoons
Markings are made so that people know what cutlery is made of. The capital letters used to mark Soviet spoons and forks represent the names of the metals that make up the alloy. The main types of brands used by industry in the USSR are presented in the table.
| MN (MELCH) | This option means cupronickel |
| MSC | A slightly different variety of the same metal with the addition of zinc. Another name is nickel silver |
| AL | This abbreviation means aluminum and its alloys |
| Stainless steel | The inscription stands for stainless steel |
Silver products are marked with 925 and 999 purity. Antique lovers are advised to be careful when handling pre-revolutionary items. At that time, cutlery was covered with only a thin layer of precious metal.
Application area
One of the important distinctive properties of cupronickel is its immunity to sea water. Therefore, the alloy is widely used in shipbuilding, making parts for marine transport and submarines. They also make thermoelements and high-precision resistors. Cupronickel is used for modern coins. Monel alloy is used as a structural material in industry.
Widely used in jewelry. The finished products look like silver. Often blackened, patinated, processed with filigree and granulation.
For several centuries, this alloy has been used to make dishes that are not inferior in beauty to silver and superior in properties:
Cupronickel cutlery - knives, forks, spoons, made in the same design, are a table decoration and an excellent purchase for your own use and as a wonderful gift for any memorable date.
Samovars, coffee Turks and cezves are coated with a tin layer on the inside, which makes them safe when exposed to high heat.
Cup holders - low thermal conductivity and durability will allow you to enjoy your favorite drink without the risk of burning your hands.
Trays, candy bowls, dishes, salt shakers and vases - beautiful products will be the highlight of the festive table and decorate the room.
Tea and coffee sets - make up various sets and items, decorated in design, for any number of people.
Wine sets - usually a set includes a jug with glasses, a tray, and sometimes a set of saucers or shot glasses.
Varieties of cupronickel spoons:
- Traditional cupronickel spoons with blackening. Holiday option. Exquisite, each item is engraved by hand. All products have a carved monogram.
- With a polished surface - for everyday use. A monogram is usually engraved on the left side of the handle, and the alloy grade is indicated on the back.
- With natural gilding - for the most special occasions. The best and most expensive products are considered to be “Kolchuginsky Melchior”. The factory's cutlery is famous for its quality. Items with silver and gilding are designed in the style of the name of the cutlery.
Brass is an eternal metal
Brass is an alloy of copper and zinc with a noble yellow color. It was first obtained in England in 1781, although it was used in ancient Rome, where copper was smelted with zinc ore.
Yellow copper
Brass is called yellow copper. It is comparatively lighter than copper. Over time, the color of gold changes, acquires a patina and loses its shine. Therefore, brass is more suitable for interior decoration. At the same time, it is more durable and wear-resistant than copper. Hard, hard to bend. It is called "eternal metal".
Brass maintains a polished surface for a long time. Can be plated with gold and tinted in any color.
Fake gold
The mechanical properties of brass L68 and L62 are similar to 583 gold. They are used as training material for jewelers. However, this feature of brass is also known to scammers who make fake “gold” jewelry from it.
Is cupronickel harmful to health?
Alloys are not only not harmful, but even beneficial to health. After all, cupronickel contains:
- Nickel – as it accumulates in the body, contributes to the long-term maintenance of low blood glucose levels and enhances the beneficial effect of the hormone insulin. Nickel ions promote better absorption of vitamin C and stabilize blood pressure.
- Copper is one of the components of human blood; it is part of enzymes and destroys pathogenic bacteria.
- Cupronickel alloy has a beneficial effect on the skin. Increases elasticity and maintains youth. With constant use of water saturated with cupronickel ions, it reduces the number of wrinkles.
Advantages and disadvantages
Cupronickel tableware has many advantages over products made from other materials. The main ones:
- slow heating, which is extremely important for appliances used in the kitchen;
- cupronickel products have a beautiful appearance;
- the material does not suffer from corrosion;
- metal does not bend, because has high strength;
- Cupronickel has a positive effect on the human body, because contains nickel and copper ions;
- the metal has a more affordable price than silver.
In addition, this material has a significant service life and has a positive effect on the human body. It is as follows:
- Nickel ions, concentrating in the human body, stabilize the glucose balance, which is extremely important for patients with diabetes;
- these compounds stabilize blood pressure in hypertension;
- copper is required by the body for the stable course of protein metabolism processes and maintaining the functioning of the musculoskeletal system;
- this microelement has a slight antibacterial effect.
Alpaca. Mexican silver replacement
Another substitute for silver is alpaca. This is an alloy of Mexican or South American origin. Alpaca is also sometimes called "the new silver" and is essentially nickel silver. .
It contains 60% copper, 20% nickel, 20% zinc and 5% tin. Silver alpaca has a slightly gray tint and a slight patina. Jewelry made from this alloy bears the mark Alpaca or Alpaca. It is sometimes used as a base metal for silver plating or counterfeit vintage jewelry.
To write the article, the books were used: “Materials Science and Technology of Artistic Processing of Metals” Flerov A.V., Moscow 1981 and “Stone That Gives Birth to Metal” Zdorik T.B., Moscow 1984.
Source