Crystal Titanium Bar
Titanium
- lightweight durable metal of silver-white color. It exists in two crystal modifications: α-Ti with a hexagonal close-packed lattice, β-Ti with cubic body-centered packing, the temperature of the polymorphic transformation α↔β is 883 °C. Titanium and titanium alloys combine lightness, strength, high corrosion resistance, low thermal coefficient expansion, ability to operate in a wide temperature range.
- Structure
- Properties
- Reserves and production
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Chromium
— structure and physical properties
STRUCTURE
Crystal crystal structure
Titanium has two allotropic modifications. The low-temperature modification, existing up to 882 °C, has a hexagonal close-packed lattice with periods a = 0.296 nm and c = 0.472 nm. The high-temperature modification has a body-centered cube lattice with a period a = 0.332 nm. The polymorphic transformation (882 °C) with slow cooling occurs according to the normal mechanism with the formation of equiaxed grains, and with rapid cooling - according to the martensitic mechanism with the formation of an acicular structure. Titanium has high corrosion and chemical resistance due to the protective oxide film on its surface. It does not corrode in fresh and sea water, mineral acids, aqua regia, etc.
Criterias of choice
Titan water heaters are a wide variety of equipment with different performance characteristics. The main one is the volume of the water tank. The second indicator is the size of the firebox. The larger the firebox, the more firewood you can put in it, the faster the water will heat up.
There are also purely specific qualities of a water heating unit that you need to pay attention to when choosing:
- if the firebox is made of cast iron rather than steel, it will last longer;
- water will heat up faster in a one-piece type boiler;
- the detachable version makes it possible to rotate the tank relative to the firebox, which allows you to accurately position the mixer relative to the place where water procedures are taken;
- a stainless steel tank will last almost forever, but such a device costs more;
- some manufacturers line the inside of the firebox with fireclay refractory bricks; this design withstands temperature conditions more effectively;
- There are wood-burning water heaters (titans) on sale, with a heating element installed in the water tank.
The latest modification is very popular. It makes it possible not to use firewood.
When you turn on the electric heating element, a large amount of electricity consumption accrues.
PROPERTIES
Titanium crystals
Melting point 1671 °C, boiling point 3260 °C, density of α-Ti and β-Ti, respectively, is 4.505 (20 °C) and 4.32 (900 °C) g/cm³, atomic density 5.71 × 1022 at/ cm³. Plastic, weldable in an inert atmosphere. Technical titanium used in industry contains impurities of oxygen, nitrogen, iron, silicon and carbon, which increase its strength, reduce ductility and affect the temperature of the polymorphic transformation, which occurs in the range of 865-920 °C. For technical Titanium grades VT1-00 and VT1-0, density is about 4.32 g/cm3, tensile strength is 300-550 MN/m2 (30-55 kgf/mm2), relative elongation is not lower than 25%, Brinell hardness is 1150-1650 MN /m2 (115-165 kgf/mm2). Is paramagnetic. Configuration of the outer electron shell of the Ti 3d24s2 atom.
It has a high viscosity and, during machining, is prone to sticking to the cutting tool, and therefore requires the application of special coatings to the tool and various lubricants.
At ordinary temperatures it is covered with a protective passivating film of TiO2 oxide, making it corrosion resistant in most environments (except alkaline). Titanium dust tends to explode. Flash point 400 °C.
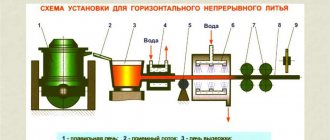
Heat treatment of titanium alloys
To improve performance, titanium alloys are subjected to heat treatment. This process becomes significantly more complicated due to the fact that the restructuring of the crystal lattice of the surface layer takes place at temperatures above 500 degrees Celsius. For VT5 and VT6-S alloys, annealing is often carried out. The holding time may vary significantly, depending on the thickness of the workpiece and other linear dimensions.
Parts made from VT14 must withstand temperatures of up to 400 degrees Celsius at the time of use. That is why heat treatment involves hardening followed by aging. In this case, hardening requires heating the environment to a temperature of about 900 degrees Celsius, while aging involves exposure to an environment with a temperature of 500 degrees Celsius for more than 12 hours.
Induction heating methods allow a wide variety of heat treatment processes to be carried out. Examples include annealing, aging, normalization, and so on. Specific heat treatment modes are selected depending on what performance characteristics need to be achieved.
RESERVES AND PRODUCTION
Titanium crystals
Main ores: ilmenite (FeTiO3), rutile (TiO2), titanite (CaTiSiO5).
As of 2002, 90% of mined titanium was used to produce titanium dioxide TiO2. World production of titanium dioxide was 4.5 million tons per year. Confirmed reserves of titanium dioxide (excluding Russia) are about 800 million tons. As of 2006, according to the US Geological Survey, in terms of titanium dioxide and excluding Russia, reserves of ilmenite ores amount to 603-673 million tons, and rutile ores - 49.7- 52.7 million tons. Thus, at the current rate of production, the world's proven reserves of titanium (excluding Russia) will last for more than 150 years.
Russia has the second largest reserves of titanium in the world, after China. The mineral resource base of titanium in Russia consists of 20 deposits (of which 11 are primary and 9 alluvial), fairly evenly distributed throughout the country. The largest of the explored deposits is located 25 km from the city of Ukhta (Komi Republic). The deposit's reserves are estimated at 2 billion tons.
Titanium ore concentrate is subjected to sulfuric acid or pyrometallurgical processing. The product of sulfuric acid treatment is titanium dioxide powder TiO2. Using the pyrometallurgical method, the ore is sintered with coke and treated with chlorine, producing titanium tetrachloride vapor, which is reduced at 850 °C with magnesium.
The resulting titanium “sponge” is melted down and cleaned. Ilmenite concentrates are reduced in electric arc furnaces, followed by chlorination of the resulting titanium slag.
Obtaining titanium
The metal source is titanium dioxide.
Its formation occurs during the processing of ilmenite. As a result, titanium slag is formed, which is subjected to further processing. Sulfuric acid is added to the concentrate, which produces titanium dioxide.
Another method is to combine with carbon (coke), chlorine and further heating in the presence of magnesium.
Calcium reduction of titanium dioxide is also used. The last process involves conducting an electric current, which leads to the decomposition of calcium oxide (oxygen at the anode and calcium itself).
Oxygen acts as an oxidizing agent, calcium, being a metal, moves to the cathode, simultaneously reducing titanium. The process happens several times. The outcome of the reaction is a titanium sponge that requires cleansing.
ORIGIN
Titanium Ore
Titanium is in 10th place in terms of prevalence in nature. The content in the earth's crust is 0.57% by weight, in sea water - 0.001 mg/l. In ultramafic rocks 300 g/t, in basic rocks - 9 kg/t, in acidic rocks 2.3 kg/t, in clays and shales 4.5 kg/t. In the earth's crust, titanium is almost always tetravalent and is present only in oxygen compounds. Not found in free form. Under conditions of weathering and precipitation, titanium has a geochemical affinity with Al2O3. It is concentrated in bauxites of the weathering crust and in marine clayey sediments. Titanium is transferred in the form of mechanical fragments of minerals and in the form of colloids. Up to 30% TiO2 by weight accumulates in some clays. Titanium minerals are resistant to weathering and form large concentrations in placers. More than 100 minerals containing titanium are known. The most important of them are: rutile TiO2, ilmenite FeTiO3, titanomagnetite FeTiO3 + Fe3O4, perovskite CaTiO3, titanite CaTiSiO5. There are primary titanium ores - ilmenite-titanomagnetite and placer ores - rutile-ilmenite-zircon. Titanium deposits are located in South Africa, Russia, Ukraine, China, Japan, Australia, India, Ceylon, Brazil, South Korea, and Kazakhstan. In the CIS countries, the leading places in explored reserves of titanium ores are occupied by the Russian Federation (58.5%) and Ukraine (40.2%).
History of metal discovery
It all started in 1791, when, independently of each other, simultaneously W. Gregor (England) and M. G. Klaproth (Germany) obtained titanium dioxide , but were unable to isolate the pure substance from it. Mineralogist and part-time rural priest Gregor studied black ferruginous sand found in the vicinity of his parish. The result was the extraction of a titanium compound - shiny grains, which were named “menakin” (from the mineral menakanite) to immortalize the Englishman’s native place.
Around the same time, the chemist Klaproth, studying red sands brought from Hungary, found a new substance in the mineral rutile and called it “titanium”. And, a few years later, he proved that rutile and menaken earth are the same compounds. In 1825, the Swedish chemist Berzelius obtained the first sample of titanium metal , but this did not allow progress in the study of properties, since impurities made the sample brittle and unsuitable for mechanical processing.
Only in 1925, Dutch chemists van Arkel and de Boer, using the thermal decomposition of titanium iodide, which was not widely used, obtained a substance with 99.9% purity. Such a metal had ductility; it could be rolled into sheets, wire and foil. This made it possible to begin a full-scale study of physical and chemical properties, attract the attention of engineers and builders, and outline areas of application. And already in 1940, the Kroll process for the reduction of titanium tetrachloride with magnesium appeared, which is still successfully used to this day.
APPLICATION
Titanium products
Titanium alloys play an important role in aviation technology, where they strive to obtain the lightest structure combined with the necessary strength. Titanium is lightweight compared to other metals, but at the same time can operate at high temperatures. Titanium alloys are used to make the casing, fastening parts, power kit, chassis parts, and various units. These materials are also used in the construction of aircraft jet engines. This allows you to reduce their weight by 10-25%. Titanium alloys are used to produce compressor discs and blades, air intake and guide vane parts, and fasteners.
Titanium and its alloys are also used in rocket science. Due to the short-term operation of engines and the rapid passage of dense layers of the atmosphere in rocket science, the problems of fatigue strength, static endurance and partly creep are eliminated to a large extent.
Due to its insufficiently high thermal strength, technical titanium is not suitable for use in aviation, but due to its exceptionally high corrosion resistance, in some cases it is indispensable in the chemical industry and shipbuilding. Thus, it is used in the manufacture of compressors and pumps for pumping such aggressive media as sulfuric and hydrochloric acid and their salts, pipelines, shut-off valves, autoclave, various types of containers, filters, etc. Only titanium is corrosion resistant in environments such as wet chlorine, aqueous and acidic chlorine solutions, therefore equipment for the chlorine industry is made from this metal. Heat exchangers are made from titanium that operate in corrosive environments, for example, nitric acid (non-smoking). In shipbuilding, titanium is used for the manufacture of propellers, plating of ships, submarines, torpedoes, etc. Shells do not stick to titanium and its alloys, which sharply increase the resistance of the vessel as it moves.
Titanium alloys are promising for use in many other applications, but their spread in technology is hampered by the high cost and scarcity of titanium.
Titanium - Ti
| Molecular weight | 47.88 g/mol |
| origin of name | The mineral got its name in honor of the Titans, characters from ancient Greek mythology, the children of Gaia. |
| IMA status | confirmed in 2010 |
Types of titanium alloys
Titanium alloys are classified according to a fairly large number of characteristics. All alloys can be divided into several main groups:
- High-strength and structural - durable titanium alloys, which also have fairly high ductility. Due to this, they can be used in the manufacture of parts subject to variable loads.
- Low-density heat-resistant alloys are used as a cheaper alternative to heat-resistant nickel alloys within a certain temperature range. The strength of such a titanium alloy can vary over a fairly wide range, depending on the specific chemical composition.
- Titanium alloys based on a chemical compound present a heat-resistant structure with low density. Due to a significant reduction in density, the weight is also reduced, and the heat resistance allows the material to be used in the manufacture of aircraft. In addition, this brand is also associated with high plasticity.
The marking of titanium alloys is carried out according to certain rules, which make it possible to determine the concentration of all elements. Let's take a closer look at some of the most common types of titanium alloys.
PHYSICAL PROPERTIES
| Mineral color | Silver gray |
| Stroke color | grayish black |
| Transparency | opaque |
| Shine | metal |
| Cleavage | No |
| Hardness (Mohs scale) | 4 |
| Kink | hackly |
| Strength | pliable |
| Density (measured) | 4.503 g/cm3 |
| Radioactivity (GRapi) | 0 |
| Magnetism | paramagnetic |