Initially, scientists thought that all combustible materials contain a special substance - “phlogiston”, supposedly because of it the combustion process occurs. However, in the 18th century, chemists discovered that in reality there was no mysterious element, and that the cause of fire combustion was oxygen. It has been experimentally proven that flame is a consequence of the interaction of a combustible material with an oxidizer.
Before we move on to combustion temperature, it is important to become familiar with the physics of the combustion process and study the phases of the process.
What is the temperature when coal burns?
Coal burns at temperatures exceeding 1100 °C. Finished coal consists mainly of carbon.
Interesting materials:
How to set up Internet on a Moldcell phone? How to set up Internet on an MTS phone? How to set up Yota Internet on a push-button phone? How to set up Yota Internet on an old phone? How to set up a camera on your phone in Skype? How to customize the keyboard on Redmi phone? How to set up a Facebook news feed on your phone? How to set up mobile internet on a ZTE phone? How to set up mts internet on your phone manually? How to set up a navigator on your phone?
Temperature
The flame temperature depends on the nature of the combustible substance and the intensity of the oxidizer supply. For example:
- The ignition temperature for most solid materials is 300 °C.
- The flame temperature in a burning cigarette is 250-300 °C.
- Match flame temperature 750-1400 °C; while 300 °C is the ignition temperature of wood, and the combustion temperature of wood is approximately 800–1000 °C.
- The combustion temperature of propane-butane is 800-1970 °C.
- The flame temperature of kerosene is 800 °C, in an environment of pure oxygen – 2000 °C.
- The combustion temperature of gasoline is 1300-1400 °C.
- The flame temperature of alcohol does not exceed 900 °C.
- Magnesium combustion temperature – 2200 °C; a significant part of the radiation is in the UV range.
Highest known combustion temperatures:
- dicyanoacetylene C4N2 5260 K (4990 °C) in oxygen and up to 6000 K (5730 °C) in ozone;
- cyanogen (CN)2 4525 °C in oxygen.
Since water has a very high heat capacity, the absence of hydrogen in the fuel eliminates heat loss due to the formation of water and allows the development of high temperatures.
Spread speed
Flame propagation through a pre-mixed (undisturbed) medium occurs from each point of the flame front normal to the flame surface. The magnitude of this normal flame propagation speed (hereinafter referred to as NFSP) is the main characteristic of a flammable environment. It represents the minimum possible flame speed. NSRP values differ for different combustible mixtures - from 0.03 to 15 m/s.
The spread of flame through real-life gas-air mixtures is always complicated by external disturbances caused by gravity, convective flows, friction, etc. Therefore, the actual flame propagation speeds always differ from normal ones. Depending on the nature of combustion, flame propagation speeds have the following ranges of values:
- deflagration combustion – up to 100 m/s;
- explosive combustion – from 300 to 1000 m/s;
- detonation combustion – over 1000 m/s.
Fire and ancient people
The controlled use of fire to provide warmth and light is one of the first great achievements of mankind. This made it possible for ancient people to explore places with harsher climates, prepare food, protect themselves from predators, and process certain materials. It has been proven that the ancestors of modern people knew how to use fire for at least 790 thousand years. Some archaeological evidence indicates its use much earlier:
- 1.6 million years ago - analysis of burnt antelope bones in a cave in South Africa confirms that they were burned by australopithecus in a man-made fire.
- 1.9 million years ago - traces of the oldest controlled fire were found in another cave on the border of the Kalahari Desert. Preliminary evidence suggests that Homo erectus has been cooking over fires since its appearance.
Fire is very important for human development, as it allowed our ancestors to cook food and keep warm
Many cultures have worshiped open flames and used them in religious rituals for thousands of years.
Fire has retained its role as an important element in many ceremonies to this day. His significance for people was so great that he became a hero of myths and the basis of ideological systems: Prometheus stole fire from the gods to give it to people; Aristotle defined it as one of the four natural elements; Chinese philosophers gave it the role of one of the five essences that make up all living things.
History[edit | edit code]
Ronson brand gasoline lighter
Modern disposable gas lighter "Cricket"
The first gas lighter, the Döbereiner flint, was invented by Johann Wolfgang Döbereiner in 1823. In it, chemically produced hydrogen was catalytically ignited on platinum. Despite the explosiveness of hydrogen and the use of caustic acid, it was produced until 1880.
There were also mechanical flints made on the basis of weapon flintlocks. Based on these ideas, Cartier received a patent for a lighter in 1867.[1] However, the significant size of the classic flint based on flint and iron did not allow making a small-sized lighter. The situation changed dramatically in 1903 with the discovery of ferrocerium by Baron Karl Auer von Welsbach. This alloy, replacing iron in steel, made it possible to replace the inconvenient mineral flint with ordinary steel. And today misch metals are the basis for the manufacture of cutting stones for lighters. Then the flint lighter acquired a design that has survived practically unchanged to this day: a jagged steel wheel strikes a spark from a ferrocerium chair, and the spark ignites a wick soaked in gasoline or gas escaping from the valve.
Austrian lighter from the 1920s
The development of lighters was accelerated during the First World War. Soldiers used matches to see the way in the dark, but the intense flash when lit gave away their location. The need for fire without a big flash fueled the development of the lighter industry. By the end of the war, lighters were a mass-produced product. The leader in the production of such lighters at that time was the homeland of ferrocerium, Austria, as well as Germany. A little later, lighters began to be mass produced around the world.
In 1947, STDuPont presented the world's first gas lighter of a modern design at an international exhibition in Paris. In 1961, the disposable “Cricket” lighter began to be sold for the first time. In the 1980s, they began to produce lighters with high vapor pressure at the outlet of the gearbox, that is, turbo lighters. They produced a sharp, directed flame that was difficult to extinguish by the wind.
Characteristics
Main characteristics:
- The combustion temperature exceeds 500 degrees;
- The flame is smoky, with the release of phenol;
- Self-extinguishing properties;
- The dusty state of polycarbonate from 700 degrees is explosive;
- The substance melts within 280-310°C;
- To increase heat resistance, the material is heated to the glass transition temperature;
- Polycarbonate is transparent;
- Does not have a specific odor;
- Non-toxic material;
- Softening starts at 220 °C.
Firewood burning phases
Wood itself does not spontaneously ignite; it must go through various phases before it begins the combustion process.
Warming up wood
Before wood can ignite, it must be heated by some heat source and reach combustion temperature. As soon as the temperature threshold of the fire exceeds 150°C, the process of evaporation of moisture from the wood and gradual charring will begin. Indeed, so-called dry wood always contains from 15 to 20% moisture.
As soon as the temperature reaches 300 °C, the surface of the wood will begin to smolder and emit white smoke. This smoke is a mixture of steam from evaporated moisture, as well as components of thermal decomposition.
At this stage, the combustion process has not yet begun, so if you stop heating, combustion will not occur.
Lighting a fire
Flue gas outbreak
At the second stage, the release of gaseous components begins to intensify. When a critical mass of pyrolysis gases is reached, a flash occurs and a fire begins. A bright yellow flame appears on the surface of the firewood, and a sharp jump in degrees occurs.
To achieve this stage, it is necessary that the pyrolysis gases be heated from 200 to 300 °C. Only at this temperature is it possible to start ignition of the pyrolysis gas.
Flash of pyrolysis gases
Wood ignition
Once the gas ignites, the wood itself ignites. Once it reaches a temperature of 450 to 650 °C (using an external heat source), not only the surface, but also the inside of the log begins to burn.
The following factors may also affect the rate of ignition:
- Wood density – small and porous firewood ignites faster than dense wood;
- Shape of firewood - massive logs burn worse than ribbed and jagged ones;
- Humidity – damp wood takes much longer to ignite because it takes time for the moisture to evaporate;
- Oxygen – a sufficient flow of oxygen is needed to keep the flame from going out.
Ignition of wood
Wood burning
If all necessary conditions are met, the flame completely covers the entire area of the wood and does not die out. This means that the ignition has entered the combustion stage. This stage has two phases - burning with a flame and smoldering.
- Flame combustion lasts as long as the pyrolysis gas burns.
- Smoldering is characterized by the gradual combustion of coal, while the pyrolysis gas is released so slowly that due to its low concentration it cannot ignite. When wood is smoldering, oxygen contributes to the further spread of the reaction to the remaining fuel.
As long as there is enough oxygen, fuel and the required temperature concentration in the combustion zone, this stage will continue.
Burning wood in a fire
It is during the combustion phase that the highest flame temperatures are observed.
Wood attenuation
As soon as support for one of the above conditions ceases, the process enters the final stage - attenuation.
The fire is dying out
Types of wood
There are several patterns that cause differences in the combustion of different types of wood. First of all, this is the presence of resins - they significantly add the calorific value of firewood. Soft wood burns more easily due to its low density. Heavy rocks maintain combustion for a long time.
While the density of wood varies significantly from type to type, their calorific value per unit mass is almost the same (with the exception of resinous softwoods). Regardless of what types of trees are used for firewood, humidity is the main factor influencing both the combustion process and the thermal result.
Knowledge of different types of wood allows you to get comfortable burning with less wood consumption
List of features of wood of some species:
- acacia - burns slowly and gives a lot of heat, dries quickly, makes a characteristic crackling noise in the fireplace;
- birch - burns quickly, ignites easily even when wet, gives an even and steady fire;
- beech is a high-calorie fuel and leaves little ash;
- oak - high calorific value, emits a pleasant smell when burning, dries for a very long time;
- poplar - low heat of combustion;
- fruit trees - burn slowly and evenly;
- conifers - aromatic smoke, can shoot resin, produce a lot of soot.
Knowing the basics of handling wood as fuel allows you to achieve comfortable combustion with less wood consumption.
It is only important not to forget the main thing: an uncontrolled open flame can be very dangerous for living beings. In addition to burns from flames and smoldering coals, fire can cause incomparably more trouble if it breaks out into a fire.
Description of the combustion process
Combustion is a chemical reaction in which large molecules are broken down into smaller, faster molecules by rearranging the bonds between atoms. The reaction releases energy in the form of heat and light. Flame is the visible process of air combustion. Fire appears as a result of an oxidation-reduction reaction. For this reaction to occur, 3 main components must be present:
- fuel: solid, liquid, gaseous (wood, coal, oil, gas and others);
- oxidizing agent: oxygen;
- activator: a trigger to start the process, for example, a spark, the sun, matches, etc.
Wood burning
Softening
Increasing degrees leads to vitrification of the material, in other words, glass transition. If you continue to heat the polyester, it will begin to soften. In this way it is possible to achieve a highly elastic state of polycarbonate. The viscosity of bisphenol A-based materials located in close proximity to the melting point is very high. For this reason, it is quite difficult to “catch” the liquid state of a substance.
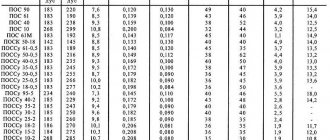
Ignition, spontaneous combustion and smoldering temperatures of some materials
Consumers completely in vain ignore such parameters as the temperatures of ignition (ignition), spontaneous combustion (spontaneous combustion) and smoldering of modern materials during the construction and renovation of premises. Ignoring them can result in great disaster: accidents and loss of property. After all, most of us only carefully study the wear resistance, strength, and specific heat capacity of building materials.
In this article we will try to fill this gap and present the auto-ignition temperatures - or more precisely, “the minimum temperatures required for the ignition of paper, gasoline, many materials, as well as gas or steam in air without the presence of a spark or flame” (all in degrees Celsius) according to foreign sources, part in the table - the rest in the text:
The lowest auto-ignition temperatures are for white phosphorus - 34 and transparent -49 (but for amorphous - 260 degrees), carbon disulfide - 90, diethyl ether - 160, acetaldehyde - 175 degrees (in degrees Celsius). Next comes a group of materials that require higher, but not prohibitive, temperatures to ignite.
Acetylene will ignite at 305, acetone and propanone at 465, bituminous coal and anthracite will glow at 464 and 600 degrees, respectively, benzene will spontaneously ignite at 560, gasoline will spontaneously ignite at 260-280 degrees (kerosene is lower at 210 o C), butadiene - 420 , butane - 405 (or 420 degrees), bitumen coal at 300, butyl acetate - 421, butyl alcohol - 345, butyl methyl ketone - 423, hydrogen -500, heptane - 204, hexane - 223, hexadecane, cetane -202, hydrogen - 500 , gas oil - 336, glycerin - 370.
Diesel fuel (foreign brand Jet A-1) ignites at 210 degrees, charcoal and coke coal - 349 and 700, respectively, dichloromethane - 600, diethylamine - 312, diisobutyl ketone - 396, diisopropyl ether - 443, dimethyl sulfoxide monoxide - 215, dodecane and dihexyl - 203, isobutane-462, isobutene-465, isobutyl alcohol-426, isooctane-447, isopentane-420, isoprene-395, isopropyl alcohol-399, isophorone-460, isohexane-264, isononane-227, isopropyl alcohol-399, light hydrocarbons - 650.
Lignite glows at 526 degrees, carbon spontaneously ignites - 609, coal oil - 580, kerosene - 295, fuel oil (depending on the brand) has a self-ignition temperature of 210-262 degrees, magnesium - 473, methane - 580, methanol, methyl alcohol - 470 (there is a brand with t=375), nitroglycerin will ignite at 254 degrees, nylons at 289-377, sulfur - 243, styrene - 490, propylene, propene - 458, polyethylene will ignite depending on the chlorine content at temperatures - 415-420 degrees, polystyrene - 226, polyvinyl alcohol - 405, propane - 455, industrial gas - 750, carbon - 700, carbon monoxide - 609, semi-anthracite coal - 400, cotton fabric - 267, cyclohexane - 245, ethylcellulose - 188 degrees Celsius.
Jet fuel A1 ignites at a temperature of 210 degrees Celsius. Popular materials now are products made of polycarbonate and polypropylene. Polycarbonate ignites at a fairly high temperature - 478, but polypropylene will ignite before paper at a temperature of 201 degrees Celsius.
People often forget to mention the ignition temperatures of rubber and rubber products. Rubber, butadiene will ignite at a low temperature of 155, and rubber, butyl at 185 degrees. The self-ignition temperature of low-purity natural rubber is 191, and high-purity rubber is 331, vulcanized rubber is 412, with the addition of butadiene-styrene, depending on the additives, 182 degrees (with 24% additive filling) and 280 degrees (with 85% additive).
READ ALSO: A unified digital platform of the Moscow Construction Complex has been created
Many people are interested in the temperature of ignition or ignition of roofing felt or roofing felt. Of course, the composition and characteristics of these materials may differ significantly. At the same time, the following ignition temperatures of roofing felt are called: 365 ° C. When stacking, thermal spontaneous combustion is easily possible, and fresh roofing material is especially at risk of spontaneous combustion.
Along the way, it is useful to touch upon the ignition temperatures of some other roofing materials. For example, roofing tiles made of bituminous asphalt. Typical ingredients of imported asphalt roofing include: limestone, oxidized asphalt, mineral granules, fiberglass mat (fiberglass and urea, formaldehyde and formaldehyde binder) and a sand and talc backing. In a typical shingle, asphalt makes up about 20% of the shingle's mass, aggregate 43%, and the surface granules 25%. So, the ignition temperature of asphalt is 400 degrees Celsius, but the softening temperature is 54-173 ° C. From here you can estimate the ignition temperature of such a roof.
In general, asphalts that will be oxidized to produce asphalt roofing products must have a minimum flash point of 260 °C (500 °F).
Like most of its products, oil ignites at a fairly low temperature - 225 degrees Celsius; for obvious reasons, the combustion or ignition temperatures of paper are very close - 218-246 degrees, peat - 227, but dry oak wood is much higher - 482 degrees and pine forest - 427, just wood - 300 degrees, semi-anthracite coal - 400. Strictly speaking, the standardized value of the ignition (ignition) temperature of paper is 233 ° C or 451 ° F ", and this must be taken into account, since paper fire is frequent fires are caused by left behind cigarette butts or unextinguished matches.
Severe hydrocarbons are self-flammable at- 750, Toluol- 535, cotton- 221, cycloglixan-245, cyclohexanol-300, cycloglopsanon-420, cyclopropopan-498, acetic acid- 427, carbon-700, furfurol-316, epihlorgidrin- 416, ethan- ethane- 515, ethylene, ethene-450, ethyl acetate-430, ethyl alcohol, ethanol-365, ethylene oxide-570 gr. Celsius.
As a result, consumers can often become unwitting victims of an accident: fire, poisoning by combustion products and smoldering materials, or, as they say, get burns out of the blue.
The following are the temperatures of ignition (ignition), spontaneous combustion (spontaneous combustion) and smoldering of some commonly used, as well as “exotic” materials that were not included in the reference material above according to domestic sources.
Note: the spontaneous combustion temperatures in the table are given for the substance in the molten state.
You also need to know about seemingly harmless spilled sugar, or rather about its dust. Any place that contains sugar dust and a lot of oxygen, such as a sugar silo, can quickly become a hazardous environment. According to fire protection studies, a room covered with at least 5 percent of its surface area in a thin layer of sugar dust (0.8 mm) poses an explosion hazard. Tiny sugar particles burn almost instantly due to the high surface area to volume ratio.
READ ALSO: Moscow will develop a new metro development program in 2021-2022
Table sugar or sucrose is highly flammable under the right conditions, just like wood.
True, at the beginning, when sugar is heated, it turns brown and caramelizes, losing moisture in it, turning almost into charcoal, and the sugar molecules line up in long chains. As the temperature rises, a flash occurs that blinds and an explosion occurs. These properties of sugar are considered by some to be a biofuel option, and more.
Well, somehow the flammability of mineral wool, which is considered and classified as non-flammable and safe, has been forgotten. But it has its “buts”... at least in relation to chimneys. Rock wool products are often used as insulation in chimney penetrations.
Mineral wool always contains an organic binder. Research has shown that smoldering of this organic material can occur in chimney insulation. Smoldering is a flameless combustion that propagates at a very low speed in a porous medium and is characterized by the release of heat. At the beginning of the smoldering process, oxidation of the environment predominates, which can occur if there is a sufficient amount of energy. In the case of chimney insulation with mineral wool, hot flue gases, which is very important, add energy to the wool. A stable process occurs if the porous material is of sufficient thickness and serves as a thermal insulator to prevent the reaction heat from being released into the environment.
Temperatures ranging from 400 to 750 °C have been measured in smoldering processes in the reaction zone. The smoldering combustion generates additional heat in the penetrating structure, which in turn increases the temperature of both the penetrating insulation and the surrounding floor and roof structures. The additional heat can have a significant impact on the chimney penetration temperature and create a potential fire hazard in surrounding structures. Organic material begins to evaporate when its temperature reaches 200 °C, and, in studies, all organic material burned when the temperature exceeded 500 °C. Similar fire retardant properties and pyrolysis of binders have been reported for stone wool.
The amount of additional heat generated by smoldering combustion depends on the amount of organic material and the maximum temperature of the insulation.
Not all materials literally burn up. There are those that, starting from a certain temperature, smolder or melt. The settled dust of coal melts at 149 degrees Celsius, organic glass at 125 degrees, aluminum at 320, even tea at 220 degrees.
In conclusion, we should provide material that can be no less useful in practice: what is the calorific value of individual types of fuel, as well as an alternative to oil and gas in terms of the high calorific value of metal filings.
How to measure the temperature of coals in a barbecue
The temperature of the coals in the grill can be determined with a certain accuracy without the use of special instruments. All you need to do is place your hand about 10 cm above the grill.
The number of seconds you can withstand will be equal to the approximate temperature of the coals:
- 1 sec – from 350 °C and above
- 2 sec. – around 300 °C
- 3 sec. – about 250 °C
- 4 sec. – 220 °C
- 5 sec. – less than 200 °C
Temperature of coals in the grill
Safety and regulations[edit | edit code]
There are international and national requirements for lighters aimed at safe handling. International standard ISO 9994:2005(E) “Lighters – Safety specification”, which describes the technical requirements for lighters and testing methods. For example, to produce a flame, a minimum of two user actions with a force of at least 15 newtons is stipulated. The maximum flame height, resistance to falling and continuous burning, resistance to ambient temperatures, requirements for warning symbols, etc. are also specified.[7]
Some regional standards, such as the European EN 13869:2002, limit the design of lighters to ensure that they are not attractive to young children. For example, made in the form of objects that are not lighters (animals, cartoon characters, lanterns, cameras, etc.), which can be mistaken by children for toys and lead to injuries, burns and fires in their hands[3][4] [5][6].
Design[edit | edit code]
Stationary flameless electric lighter
Kitchen gas lighter
The design of a lighter directly depends on its purpose. The most widespread are pocket and kitchen lighters. Sometimes there are stationary lighters.
Kitchen lighters are designed for igniting household gas appliances and fireplaces. These lighters have an elongated nose so that you can get close to the burners.
Pocket lighters are small in size and easy to carry. The design is absolutely any, but the sizes are limited. Table lighters are quite rare. Such lighters are quite massive and are not designed to be carried. The design of such lighters can be any. There are also special fireplace lighters; while they are long, they have a small width and thickness, and even lighters from well-known brands. Not so long ago, touch lighters appeared, in which gas ignition occurs without mechanical influence, but by acting on a touch sensor.
Stationary lighters are usually reserved for bars and smoking rooms - they are more difficult to steal. Sometimes they become an element of the interior. There are stationary flameless lighters for smoking rooms in hazardous industries and nursing homes.
Advertising[edit | edit code]
Recently, so-called advertising lighters have become increasingly popular. They are an ordinary pocket lighter with information, usually of an advertising nature, applied using silk-screen or pad printing. Widely used by large chain stores and hotel and restaurant companies to advertise services and promote goods.
Ban on souvenir lighters[edit | edit code]
The EU and a number of US states have adopted or are preparing to adopt legislation prohibiting the circulation of souvenir lighters made in the form of objects that are not lighters (animals, cartoon characters, lanterns, cameras, etc.), which can be mistaken by children for toys and lead to injuries, burns and fires in their hands[3][4][5][6].