Device
The acetylene gas cylinder is made from seamless pipes in accordance with GOST 949-73 . Wall thickness 7 – 8 mm. The ends of the container are spherical. The top part has a hole into which the valve is screwed. A cylindrical shoe is placed on the lower part, giving the container stability.
The neck in acetylene cylinders is wider than in others where gas is stored. Through it, the container is filled with a porous mass: cast porous filler, activated birch carbon and basalt fiberglass. The loose substance absorbs acetone well and promotes uniform dissolution of acetylene in it.
Foreman of the acetylene cylinder filling section of the SpetsBallonMash plant Kurnikov A.I.: “The Americans fill the inside of an acetylene cylinder with asbestos cord. At the end of the last century, the Russian Federation abandoned such material as carcinogenic. Small particles of asbestos evaporate along with argon. In Russia, they switched to natural materials that absorb acetone: silk, leather, mineral wool, sawdust. Currently, some manufacturers use asbestos in small quantities as an additive to quartz sand and calcium hydroxide. In Germany, charcoal is mixed with magnesium carbonate and kieselguhr. When purchasing a cylinder, you should pay attention to its filling. Asbestos causes lung cancer."
Acetylene cylinders
Acetylene cylinder device
An acetylene cylinder is a universal container for storing and transporting acetylene. The cylinder body is made of seamless pipes in accordance with GOST 949-73. A hot shoe is attached to the lower part of the body, giving stability to the cylinder in a vertical position. A valve is screwed into the upper spherical part of the neck for filling and extracting gas. In the non-operating position, the valve is a shut-off device.
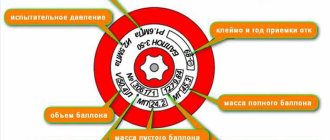
The cylinders are equipped with valves VBA-1 according to TU 26-05-527-82 (with a membrane seal) or BA-I according to TU 6-21-23-84 (with an ebonite seal). A threaded ring is pressed onto the outer part of the neck for screwing on the safety cap. At the point where the cylindrical part of the cylinder transitions into the spherical part, the following data is stamped:
- Manufacturer's mark and cylinder number;
- Date of manufacture of the cylinder;
- Working and test pressure in kgf/cm2;
- Cylinder capacity in liters;
- Tare weight (weight of the cylinder body with shoe and valve, porous mass and acetone);
- The sign of the factory that filled the cylinder with porous mass and acetone, and the date of filling;
- Brand of the filling station, date (month and year) of the survey performed and year of the next survey;
- Year and month of the porous mass inspection, stamp of the filling station and stamp “Pm”
Cylinders must be painted white with the exception of the branding area, which must be covered with colorless varnish and surrounded by a red frame. On the cylindrical part of the cylinder there must be the inscription “ACETYLENE” painted in red paint. The painting of cylinders and the inscription on them can be done with oil, enamel or nitro paints. The inscription on the cylinders must be at least 1/2 of the circle, and the height of the letters must be at least 60 mm
An acetylene cylinder is filled with porous filler and filled with acetone
The role of the porous filler:
- Protecting the acetylene cylinder from flashback or possible explosive decomposition of acetylene.
- Promotes a more uniform distribution of the solvent in the cylinder.
Depending on the porous filler, acetylene cylinders are divided into cylinders with bulk porous mass (BAU-A coal) and cylinders with cast porous mass (LPM). BAU-A coal is black grains without mechanical impurities, produced in accordance with GOST 6217-74. The cast porous mass is a cast porous block of gray color, produced according to TU 6-21-38-85 “Cylinders for dissolved acetylene with cast porous mass.”
The weight of the stuffed porous mass is 280-310 g per 1 liter of cylinder body capacity or 30% of its volume. The cast porous mass of LPM TU 6-21-38-85 is formed as a result of a hydrothermal reaction between silicon dioxide, calcium oxide hydrate and additives at elevated pressure and temperature directly in the cylinder, as a result of which a continuous cast porous block is formed in it.
Due to safety conditions, it is impossible to allow pressure in acetylene cylinders significantly exceeding 25 kg/cm2. As a result, the porous mass is impregnated with acetone, which significantly increases gas absorption, since it is a good solvent for acetylene.
For acetonation of cylinders, technical acetone GOST 2768-84 grade 1 is used.
Acetone is a colorless, flammable liquid with a characteristic odor. Self-ignition temperature 465? C. A mixture of acetone vapor with air is explosive: the explosion limits in volume fractions of acetone are the lower limit 2.2; top - 13. Liquid acetone causes skin irritation. Acetone vapors cause irritation and disease of the upper respiratory tract. Acetone is introduced into the cylinder at the rate of 225-230 g per liter of cylinder capacity. The volume occupied by acetone in the cylinder is 25-30%. To avoid excessive increase in pressure in the cylinder, part of the porous mass should not be filled with acetone. The remaining unfilled volume (gas cushion) contains compressed acetylene gas, saturated with acetone vapor; this volume is 16%.
Permissible pressure of acetylene in cylinders depending on temperature:
| Temperature, оС | -5 | 0 | +5 | +10 | +15 | +20 | +25 | +30 | +35 | +40 |
| Pressure no more than, kgf/cm2 | 13,4 | 14 | 15 | 16,5 | 18 | 19 | 21,5 | 23 | 26 | 30 |
Features of the operation of cylinders with BAU-A and LPM
The fundamental difference in the operation of acetylene cylinders with BAU-A and LPM coal is that when discharging and filling cylinders, it is not recommended to place cylinders with BAU-A coal for 5 kg of acetylene and LPM for 7 kg of acetylene on the discharge or filling ramp at the same time at 40 liter bottle.
When simultaneously filling cylinders designed for 5 and 7 kg of acetylene in a cylinder, it is almost impossible to achieve gas collection capacity of 7 kg of acetylene in a cylinder with a LPM, since cylinders filled with 5 kg, increasing the pressure in the ramp, prevent further filling of cylinders with 7 kg of acetylene.
With the simultaneous discharge of cylinders with different gas collection rates, a flow of acetylene from cylinders with a higher gas collection rate to cylinders with a lower acetylene content is possible.
Requirements for cylinders
The main reasons why cylinders are not allowed to be filled:
- The presence of cracks and dents on the cylinder body;
- Malfunction of the valve (the square of the stem is worn out, the valve is bent, the number of visible threads on the screwed-in valve is less than 2 or more than 5; gas leakage through the stuffing box, which cannot be eliminated by tightening the nut);
- Missing or poorly fitting shoe;
- More than 30% of the painted surface of the cylinder is damaged, the inscription “ACETYLENE” is missing;
- The date of inspection of cylinders has expired;
- The porous mass inspection date has expired;
- Signs that the cylinder was on fire;
- Signs of strong heating of the cylinder;
- Complete or partial blockage in the cylinder valve;
- If the weight of the cylinder with the gas remaining in it exceeds the container weight and does not agree with the calculated data;
- There are no established stamps.
In cylinders suitable for filling, it is necessary to measure the residual gas pressure; it should be no more than 1 kgf/cm2.
After measuring the residual pressure, the cylinder is weighed on a scale to determine the amount of missing acetone. By external inspection, it is necessary to determine the condition of the gaskets in the annular recesses of the valves, replace worn ones, and only after this the cylinder can be connected to the filling ramp.
New acetylene cylinders and cylinders received from the consumer with a residual pressure below 0.5 kgf/cm2 must be washed with acetylene by filling them three times to a pressure of 20 kgf/cm2 and then releasing them to a residual pressure of 0.5 kgf/cm2.
All acetylene cylinders are subject to periodic inspection every 5 years (the next inspection expires on the 15th of the month in which the test was carried out). The test is carried out with a nitrogen pressure of 35 kgf/cm2. The condition of the porous mass is checked after 24 months and at each inspection.
Refueling
Acetylene is produced by combining water and carbide . Acetylene cylinders are filled through the neck, determining its quantity by weight. Standard full cylinder 65 kg, empty 53 – 58 kg. The weight of the container with filler is indicated at the marking on the neck and is determined by weighing.
The acetone partially evaporates along with the gas. At each refill, add 130 - 150 ml.
If you fill a 5 liter container from a 40 liter cylinder, the gas in it will run out very quickly. In order for acetylene to dissolve in acetone, high pressure must be created. When pumping at home, it is impossible to create it .
Cylinder explosion
The main disadvantage of acetylene is its explosiveness. Gas can detonate for many reasons:
- achieving a critical mass of gas;
- high pressure;
- residue in the flammable gas cylinder;
- contact with lubricant or calcium carbide;
- electrification of the neck by the gas itself, passing at high speed;
- heat;
- leakage and air connection;
- impacts on the weakened walls of the container.
The gas becomes explosive if it is collected in large volumes and when the acetylene pressure in the cylinder exceeds 2 kg/cm2. To reduce the risk of spontaneous explosion, a special porous mass is placed inside the gas container. It divides all the gas into small particles, allowing it to move freely. Pure acetylene can be pumped with a maximum pressure of 25 kg/cm2.
Acetone dissolves acetylene in quantities exceeding its own weight by 10 times. Impregnation of the porous filling with acetone and subsequent filling with acetylene reduces the likelihood of an explosion to almost zero . In this case, acetone is used repeatedly. After the acetylene evaporates, it remains in the porous material and is suitable for dissolving the next batch of gas.
Seeping under the lid, acetylene is mixed with oxygen and an explosive hot mixture is obtained when a certain proportion of it with oxygen is reached.
Important! A drop of oil on the neck may cause an explosion if acetylene comes into contact with it. It is dangerous to use too much gas. When quickly moving through the neck, the metal is electrified, creating a discharge that acts as a detonator.
Oxygen cylinders
For gas welding and cutting, oxygen is delivered in steel oxygen cylinders of type 150 and 150 L. The oxygen cylinder is a steel seamless cylindrical vessel 3, having a convex bottom 1, onto which a shoe 2 is pressed; At the top, the cylinder ends with a neck 4. The neck has a conical hole into which a shut-off valve 5 is screwed in. A safety cap 6 is screwed onto the neck to protect the valve.
The most widely used for gas welding and cutting are cylinders with a capacity of 40 dm3. These cylinders have the following dimensions: outer diameter - 219 mm, wall thickness - 7 mm, height - 1390 mm. The weight of the cylinder without gas is 67 kg. They are designed for a working pressure of 15 MPa, and a test pressure of 22.5 MPa.
To determine the amount of oxygen in the cylinder, you need to multiply the capacity of the cylinder (dm3) by the pressure (MPa). For example, if the capacity of a cylinder is 40 dm3 (0.04 m3), pressure is 15 MPa, then the amount of oxygen in the cylinder is 0.04x15 = 6 m3.
Figure 1 — Oxygen cylinder
At the welding station, the oxygen cylinder is installed in a vertical position and secured with a chain or clamp. To prepare an oxygen cylinder for operation, unscrew the cap and fitting plug, inspect the valve to determine if there is any grease or oil on it, carefully open the cylinder valve and blow through its fitting, then close the valve, inspect the union nut of the reducer, attach the reducer to the cylinder valve , set the operating oxygen pressure with the adjusting screw of the gearbox. When you finish withdrawing gas from the cylinder, you must ensure that the residual pressure in it is not less than 0.05-0.1 MPa.
When handling oxygen cylinders, it is necessary to strictly observe operating and safety rules, which is due to the high chemical activity of oxygen and high pressure. When transporting cylinders to the welding site, you must firmly remember that it is prohibited to transport oxygen cylinders together with flammable gas cylinders. If the oxygen cylinder valve freezes, you need to warm it up with a rag soaked in hot water.
The reasons for the explosion of oxygen cylinders may be contact with fat or oil on the valve, falling or hitting the cylinders, the appearance of a spark when too much gas is drawn off (the neck of the cylinder is electrified), heating of the cylinder by some heat source, as a result of which the gas pressure in the cylinder will become higher than permissible.
Table 1 - Types of cylinders for liquefied gases
| Cylinder type | Pressure, MPa | Tensile strength, MN/m2 | Relative extension, % | ||
| conditional | hydraulic | pneumatic | |||
| 100 | 10 | 15,0 | 10 | 650 | 15 |
| 150 | 15 | 22,5 | 15 | 650 | 15 |
| 200 | 20 | 30,0 | 20 | 650 | 15 |
| 150L | 15 | 22,5 | 15 | 900 | 10 |
| 200L | 20 | 30,0 | 20 | 900 | 10 |
How much is 40l?
The volume of acetylene in a fully filled gas container is calculated using a simple formula:
40 × 15 × 9.2 = 5520 l;
Where: 40 l – cylinder volume;
15 kg/cm2 – pressure;
9.2 gas solubility coefficient in acetone;
5520 l = 5.5 m3 amount of acetylene.
This formula calculates the amount of acetylene that can be filled into any size cylinder. In the formula, only 40 – the number of the container’s volume – changes to 5, 10 and others .
Application of acetylene
As mentioned earlier, such a compound has increased reactivity.
Accordingly, it is used to synthesize various materials. This can be rubber, ethyl alcohol, carbon black, etc. Acetylene is also used in the production of explosives, rocket engines and lighting equipment. We have already mentioned the fact that when a substance is burned, a significant amount of heat is generated. It is for this reason that acetylene is often used in the process of cutting and welding various metals. The main competitor of acetylene in this area is propane-butane. The latter type of gas is cheaper, but it produces a low combustion temperature.
Cylinders or generators can be used to supply gas during the welding process. The first option is considered more preferable. Standard cylinders designed for 40 liters are recognized as the optimal product. They are painted white. At the same time, “Acetylene” is written on the surface in red.
It is worth noting that only dissolved and gaseous technical acetylene is suitable for gas-flame processing.
itself is filled with a special porous mass , pre-impregnated with acetone. This layer performs 2 important functions.
- Increased safety during work. Thus, the likelihood of fire and explosion spreading over a large area is reduced.
- Increasing the amount of acetylene and accelerating the process of its release. This is possible due to the provision of a significant contact surface between the gas and acetone.
What's the pressure?
According to GOST, pressure is determined at a temperature of + 20⁰C. Limit value 19 kgf/cm2. Typically, acetylene in cylinders has 14 - 16 kgf/cm2.
Gas pressure depends on temperature changes. For example, 19 kgf/cm2 at 20⁰ when cooled to -4, a total of 13.4 kgf/cm2, at 35⁰ a critical value of 26 kgf/cm2 is reached. Therefore, a pressure reserve is created in case of heating.
You can determine the amount of gas filled by a simple calculation. You need to subtract the weight of the empty cylinder from the weight after refueling and multiply by 1.09 - that’s how much a liter of gas weighs.
Calculation of oxygen in cylinders
The parameters and dimensions of oxygen cylinders can be viewed according to GOST 949-73 “Small and medium steel cylinders for gases at Рр ≤ 19.7 MPa”. The most popular are cylinders with volumes of 5, 10 and 40 liters.
According to GOST 5583-78 “Gaseous technical and medical oxygen” (Appendix 2), the volume of gaseous oxygen in a cylinder (V) in cubic meters under normal conditions is calculated by the formula:
V = K1•Vb,
Vb — cylinder capacity, dm3;
K1 - coefficient for determining the volume of oxygen in the cylinder under normal conditions, calculated by the formula
K1 = (0.968P + 1) * *
P - gas pressure in the cylinder, measured by a pressure gauge, kgf/cm2;
0.968 — coefficient for converting technical atmospheres (kgf/cm2) into physical ones;
t is the gas temperature in the cylinder, °C;
Z is the oxygen combustion coefficient at temperature t.
The values of the K1 coefficient are given in Table 4, GOST 5583-78.
Let's calculate the volume of oxygen in the most common cylinder in construction: a volume of 40 liters with a working pressure of 14.7 MPa (150 kgf/cm2). Coefficient K1 is determined according to Table 4, GOST 5583-78 at a temperature of 15°C:
V = 0.159 • 40 = 6.36m3
Conclusion (for the case under consideration): 1 oxygen cylinder = 40l = 6.36m3
Table 4. GOST 5583-78.
| Gas temperature in the cylinder, °C | The value of the Ki coefficient at excess pressure, MPa (kgf/cm2) | ||||||||||||||
| 13,7 (140) | 14,2 (145) | 14,7 (150) | 15,2 (155) | 15,7 (160) | 16,2 (165) | 16,7 (170) | 17,2 (175) | 17,7 (180) | 18,1 (185) | 18,6 (190) | 19,1 (195) | 19,6 (200) | 20,1 (205) | 20,6 (210) | |
| -50 | 0,232 | 0,242 | 0,251 | 0,260 | 0,269 | 0,278 | 0,286 | 0,296 | 0,303 | 0,311 | 0,319 | 0,327 | 0,335 | 0,342 | 0,349 |
| -40 | 0,212 | 0,221 | 0,229 | 0,236 | 0,245 | 0,253 | 0,260 | 0,269 | 0,275 | 0,284 | 0,290 | 0,298 | 0,305 | 0,312 | 0,319 |
| -35 | 0,203 | 0,211 | 0,219 | 0,226 | 0,234 | 0,242 | 0,249 | 0,257 | 0,264 | 0,272 | 0,278 | 0,286 | 0,293 | 0,299 | 0,306 |
| -30 | 0,195 | 0,202 | 0,211 | 0,217 | 0,225 | 0,232 | 0,239 | 0,248 | 0,253 | 0,261 | 0,267 | 0,274 | 0,281 | 0,288 | 0,294 |
| -25 | 0,188 | 0,195 | 0,202 | 0,209 | 0,217 | 0,223 | 0,230 | 0,238 | 0,243 | 0,251 | 0,257 | 0,264 | 0,270 | 0,277 | 0,283 |
| -20 | 0,182 | 0,188 | 0,195 | 0,202 | 0,209 | 0,215 | 0,222 | 0,229 | 0,235 | 0,242 | 0,248 | 0,255 | 0,261 | 0,267 | 0,273 |
| -15 | 0,176 | 0,182 | 0,189 | 0,196 | 0,202 | 0,208 | 0,215 | 0,221 | 0,227 | 0,234 | 0,240 | 0,246 | 0,252 | 0,258 | 0,263 |
| -10 | 0,171 | 0,177 | 0,183 | 0,189 | 0,195 | 0,202 | 0,208 | 0,214 | 0,220 | 0,226 | 0,232 | 0,238 | 0,244 | 0,250 | 0,255 |
| -5 | 0,165 | 0,172 | 0,178 | 0,184 | 0,190 | 0,195 | 0,202 | 0,207 | 0,213 | 0,219 | 0,225 | 0,231 | 0,236 | 0,242 | 0,247 |
| 0 | 0,161 | 0,167 | 0,172 | 0,179 | 0,184 | 0,190 | 0,196 | 0,201 | 0,207 | 0,213 | 0,219 | 0,224 | 0,229 | 0,235 | 0,240 |
| +5 | 0,157 | 0,162 | 0,168 | 0,174 | 0,179 | 0,185 | 0,190 | 0,196 | 0,201 | 0,207 | 0,212 | 0,217 | 0,223 | 0,228 | 0,233 |
| +10 | 0,153 | 0,158 | 0,163 | 0,169 | 0,174 | 0,180 | 0,185 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,217 | 0,222 | 0,227 |
| +15 | 0,149 | 0,154 | 0,159 | 0,165 | 0,170 | 0,175 | 0,180 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,216 | 0,221 |
| +20 | 0,145 | 0,150 | 0,156 | 0,160 | 0,166 | 0,171 | 0,176 | 0,181 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 | 0,215 |
| +25 | 0.142 | 0,147 | 0,152 | 0,157 | 0,162 | 0,167 | 0,172 | 0,177 | 0,182 | 0,186 | 0,191 | 0,196 | 0,201 | 0,206 | 0,210 |
| +30 | 0,139 | 0,143 | 0,148 | 0,153 | 0,158 | 0,163 | 0,168 | 0,173 | 0,177 | 0,182 | 0,187 | 0,192 | 0,196 | 0,201 | 0,206 |
| +35 | 0,136 | 0,140 | 0,145 | 0,150 | 0,154 | 0,159 | 0,164 | 0,169 | 0,173 | 0,178 | 0,182 | 0,187 | 0,192 | 0,196 | 0,201 |
| +40 | 0,133 | 0,137 | 0,142 | 0,147 | 0,151 | 0,156 | 0,160 | 0,165 | 0,170 | 0,174 | 0,178 | 0,183 | 0,188 | 0,192 | 0,196 |
| +50 | 0,127 | 0,132 | 0,136 | 0,141 | 0,145 | 0,149 | 0,154 | 0,158 | 0,163 | 0,167 | 0,171 | 0,175 | 0,180 | 0,184 | 0,188 |
Cylinder color
Externally, unpainted acetylene containers differ only in the width of the neck. They have a diameter of 210 mm and a standard support shoe. After painting with enamel in preparation for work, the acetylene cylinder is white . In the upper quarter of the cylindrical part the word “Acetylene” is written in red. Letters 60 - 100 mm high should occupy at least half the circumference of the container.
There is a marking on the cone near the neck. It includes:
- manufacturer's trademark;
- factory mark;
- individual number;
- brand of the organization that carried out the technical examination;
- date of manufacture;
- year of next maintenance;
- operating pressure;
- capacity in liters;
- weight in kg.
The marking is stamped onto clean metal, outlined around the perimeter with red paint and coated with varnish to protect the surface from corrosion..
Storage of acetylene - general provisions.
Due to the fact that acetylene is explosive not only when mixed with air or oxygen, but also in a pure state, there is a possibility of the cylinder exploding if a backfire occurs or if it is accidentally heated by a burner.
Acetylene is stored in transport and small-capacity cylinders of a special design. To prevent the spread of a blast wave in an acetylene cylinder, it is filled with a special cast porous mass, which most often is activated carbon, pumice, or asbestos fiber. Acetylene pumped under pressure fills all the pores of the material; to increase the gas volume, the filler is impregnated with acetone, which increases the absorption of the material. For example, up to 13 liters of acetone are poured into a transport acetylene cylinder, one liter of which can hold up to 23 liters of gas. In total, the transport cylinder can hold up to 6 kilograms of acetylene. This design of the cylinder allows the filler to dampen the resulting shock waves from an acetylene explosion, which significantly increases work safety.
Cylinders for acetylene storage are painted with white enamel, the inscription “Acetylene” is applied to it, under it are the letters “LM”, which indicates that the cylinder is filled with a cast porous mass. These inscriptions must be red.
Valve
The locking device is made of steel. With prolonged contact with copper and bronze, a chemical reaction occurs and acetylenide is formed - a combination of copper with carbon and the release of hydrogen. Copper acetylene can cause an explosion .
The valve is connected to the neck with a clamp. The thread on the fitting is left - counterclockwise.
There are no taps usual for other gas tanks. The valve is opened with a special socket wrench by rotating a square spindle located in an axis along the entire length of the body. The clamp pressure screw has a small hole on one side for the outlet fitting.
Valve device
The cast body has a through hole of variable cross-section along the axis. It contains a rod, the upper part of which is rotated with a key. At the bottom it has an ebonite seal that blocks the gas outlet.
When the spindle rotates, the hole at the bottom opens. The gas rises and exits from the side through an attached fitting. The upward exit along the spindle is blocked by leather and steel rings, which are pressed with a steel nut.
The housing fitting is sealed with a leather gasket, which is located in the recess around the hole and serves as a stop for the end of the screwed fitting.
Storage
Oxygen and acetylene cylinders are stored separately from each other. The conditions are the same:
- containers must stand vertically;
- place the cylinders in special stands and fix them;
- the room temperature should not exceed 25⁰C;
- should not be placed near heating devices or open flames;
- batches at the warehouse should not exceed 20 pieces.
Valves on containers with argon in the non-working position must be closed with special caps. As for acetylene cylinders, they are also sorted by volume.
You cannot keep a container nearby that is larger or smaller than the main group.