What is carbon dioxide
Carbon dioxide is also known as E290 (in the International Registration of Food Additives). In high concentrations it has a sour taste and odor, density 1.977 g/l. Used as an acidity regulator, antioxidant, preservative. It is a non-toxic (in acceptable doses), inert, non-flammable, heavy colorless gas, also referred to as carbon dioxide, carbon dioxide/monoxide, carbonic anhydride (CO₂).
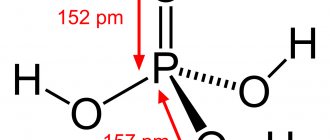
Structure of a carbon dioxide molecule:
Carbon dioxide molecule
Physical properties
Carbon dioxide exists in liquid, gaseous and solid states, in the form of white “dry ice”. The peculiarity is the rapid transition from the crystallized state to the gaseous state, bypassing the liquid stage. The color and smell are noticeable only in conditions of high CO₂ concentration. Carbon dioxide gas is heavier than air and is resistant to thermal effects, so under normal atmospheric conditions it evaporates rather than melts. Sublimates at 78°C. It does not dissolve in liquid and is partially in contact with it.
Formula and properties
Traditional formula of carbon dioxide: CO2, mass: 44 g/mol.
Inert oxide is classified as oxide acid.
- in contact with H₂O, it forms an unstable, instantly disintegrating, interconvertible acid: CO₂ + H₂O ↔ CO₂ × H₂O (dissolution) ↔ H₂CO₃;
- the reaction between the lime liquid and carbon dioxide forms calcium carbonate (the whitish sediment at the bottom of the liquid): CO₂ + Ca(OH)2 = CaCO₃↓ + H₂O;
- thermal action decomposes dioxide into oxygen and carbon dioxide: 2CO₂ = 2CO + O₂;
- CO₂ gas reacting with active metal peroxides decomposes into oxygen and carbonates: 2CO₂ + 2Na₂O₂ = 2Na₂CO₃ + O₂↑.
- CaO + CO₂ = CaCO₃;
- Al₂O₃ + 3CO₂ = Al₂(CO₃)3;
- CO₂ + NaOH = NaHCO₃;
- CO₂ + 2NaOH = Na₂CO₃ + H₂O;
- CO2 + 4H2 = CH4 + 2H2O (t°, kat = Cu2O);
- CO2 + 2Mg = C + 2MgO (t°);
Therefore, carbon dioxide has 4 important properties:
- Reacts with bases and basic oxides, forming carbonic acid salts.
- When exposed to high temperatures and reducing agents, oxidizing ability is manifested. You can restore the gaseous form with coal.
- Magnesium burning in the air continues to burn in an atmosphere of carbon dioxide.
- A qualitative reaction caused by the appearance of a white precipitate in limewater, which disappears when the dioxide is passed through the limestone liquid for a long time. As a result, calcium carbonate, deprived of the ability to dissolve, turns into soluble bicarbonate.
Properties allow carbon dioxide to have several forms. For example: at traditional atmospheric pressure, the element exists in the form of a gas. Cooling by the ocean depths transforms CO₂ into a liquid state, and freezing crystallizes it, forming ice. Sudden heating stimulates evaporation and restoration of the gaseous form.
Carbon dioxide in the atmosphere and nature
In the environment, CO₂ gas is formed by several sources: nature, humans, animals:
- people and animals inhale oxygen for life, and as a result of processing by the pulmonary system, carbon monoxide is excreted.
The same goes for plants. During photosynthesis, they absorb O₂ and remove CO2, so you should not place many flowers in an unventilated room; Important! The human body releases 0.03% carbon into the atmosphere per day = 1 kg CO2
- carbon is a component of volcanic gases, therefore, in areas with increased volcanic activity, carbon dioxide can get from the depths to the surface of the earth through cracks, soil faults, etc. In some cases, the concentration of CO2 is so high that it provokes poisoning and death of small animals;
- rotting, burning and decomposition of organic matter. Carbon dioxide is stored in the bowels of the earth, in the form of peat, coal, oil, limestone, and is also released when wood burns.
The formation of carbon dioxide gas in the bowels of the earth and the release of CO2 into the atmosphere by living beings are interconnected. Initially, organic substances (excreted by animals, limestones) are mineralized, turning into fossils, stored in the bowels of the earth for centuries. Then coal, oil, and peat are used by processing stations and release CO2 into the atmosphere.
Also, people, animals, plants, absorbing oxygen, release carbon dioxide gas into the air, which dissolves in the seas and oceans, turning into limestone.
It is believed that excess carbon dioxide is the cause of climate change in the world, because it provokes the greenhouse effect. Consequently, solar radiation easily reaches the soil surface and is retained in the atmosphere. A thermal effect occurs, melting glaciers and raising sea levels.
Consequences of climate change and increased amounts of CO2:
- evolution of plants, animals;
- global temperature rise;
- natural disasters, fires.
Humanity will suffer the most: the territory for life will decrease, which will become less comfortable. The way to save yourself is to reduce carbon dioxide emissions by minimizing the production of carbon dioxide in production and switching to environmentally friendly transport.
Origin
The formation of carbon dioxide is natural. The bulk of Earth's carbon is stored for millions of years in secure stores of carbonate rocks, such as those that make up the famous "White Cliffs of Dover" on the English Channel coast.
These rocks were created by creatures a thousand times smaller than the head of a pin. Trillions of these microscopic creatures are single-celled algae.
Volcanoes, hot springs and geysers released CO2 into the atmosphere, and the oceans slowly absorbed it. For hundreds of millions of years, single-celled algae dissolved carbon dioxide and formed tiny shells from it. The abundance of these shells formed huge deposits of chalk or limestone on the ocean floor. As a result of the shifting of tectonic plates, the earth lifted the seabed from the depths and carved out the great "White Cliffs of Dover". Polyps and algae, which can extract lime from water, used carbon dioxide to build giant coral reefs. The oceans themselves turned absorbed carbon dioxide into limestone, without the help of living beings.
Ocean absorption of CO2
Over time, barely noticeable traces of CO2 have remained in the planet's atmosphere; the concentration of carbon dioxide currently amounts to about 0.04 percent of the total air volume. Now, along with nitrogen (N2), oxygen (O2) and argon (Ar), carbon dioxide forms an integral part of the planetary air that we breathe.
In mass equivalent, there are 760 milligrams of CO2 per cubic meter of air. However, this is the difference between a barren wasteland and the riot of life in the earth's garden. In the complete absence of carbon dioxide, the Earth would turn into an ice ball. If it were doubled, namely 8 molecules for every 10,000, we would become uncomfortable in such heat.
Obtaining carbon dioxide
In addition to producing CO₂ gas using natural methods, it is also produced in the laboratory and in industry for use in the food, medical and other industries.
Laboratory methods
Laboratories produce small batches of carbon dioxide by displacing air through the interaction of salt, marble, chalk with hydrochloric acid:
CaCO₃ + 2HCl → CaCl₂+ H₂O + CO₂, NaHCO₃+ HCl → NaCl + H₂O + CO₂
Industrial production
Carbon dioxide is obtained by separating nitrogen, oxygen, and argon during the combustion of industrial waste (smoke). This is the most profitable way to produce carbon dioxide gas. Monoethanolamine is adsorbed by feeding it into the chimney. The particles absorb carbon dioxide and are fed into special tanks, where they are released (from monoethanolamine) at a certain pressure and temperature. This produces carbon dioxide in gaseous form.
Carbon oxide is produced in a liquid state by fermenting raw materials (in the production of alcohol). Then the resulting gases are treated with coal, potassium permanganate and hydrogen.
Carbon is extracted in dry form at distilleries and breweries. A complex and lengthy process performed in several stages:
- The released gas (as a result of fermentation of the raw material) is sent for washing.
- After cleaning, it penetrates into the device, where it is exposed to high pressure.
- It is served in refrigerators and cooled to a liquid state.
- Re-cleaned with charcoal.
- It is sent to refrigerators to acquire a solid form.
The resulting high-quality carbon dioxide is sent to enterprises for further use, as a coolant, in the form of snow-white “dry ice”.
Chemical reactions with carbon dioxide, compounds
Carbon itself is not flammable or active, but it reacts with other substances, making it possible to obtain new substances used in almost all areas of industry.
- The reaction between ammonia and carbon dioxide leads to the decomposition of the elements to ammonium bicarbonate: 2NH₃ + CO₂ + H₂O = NH₄HCO₃ Bicarbonate salt, widely used by bakeries.
- When ammonia and carbon dioxide interact at temperatures above 130°C and a pressure of 200 atoms, urea is produced: 2NH₃ + CO₂ → (NH₂)2CO + H₂O
- Zinc oxide is obtained by combining zinc and carbon dioxide under the influence of a temperature of 800°C.
- CO₂ gas is combined with barium hydroxide to produce hydrogen oxide with barium carbonate: Ba(OH)2+CO₂ = BaCO₃ + H₂O This produces a medium salt used to make synthetics, kitchen tiles, fireworks, red bricks, etc.
- Since carbon dioxide is not flammable, it can only support combustion in combination with magnesium: 2Mg + CO₂ = C + 2MgO The resulting magnesium oxide is used in cosmetology and as an active food additive.
- Carbon dioxide reacts with carbonates and bicarbonates to form bicarbonates: CO₂ + NaOH → NaHCO₃. More sodium forms sodium carbonate and water: CO₂ + 2NaOH → Na₂CO₃ + H₂O
- The combination of carbon dioxide and alkali serves to harden the limestone: Ca(OH)2 + CO₂ → CaCO₃ + H₂O
- When carbon dioxide and water interact, glucose is formed, which allows plants to eat (taking part in photosynthesis): 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
- Soda is obtained by combining ammonia, carbon dioxide and sodium chloride: NaCl + CO₂ + NH₃ + H₂O → NaHCO₃ + NH₄Cl
- Sodium phenolate in contact with carbon decomposes, forming a precipitate of poorly soluble phenol.
Noble gases: xenon, argon, helium, etc. do not react with carbon dioxide due to the incompatible molecular structure of these gases.
Opening
Joseph Black
The discoverer of carbon dioxide is the Scottish physicist and chemist Joseph Black. In 1756, a scientist conducted an experiment by heating white magnesia (MgCO3). As a result of heating, he revealed that magnesium carbonate decomposed to burnt magnesia (magnesium oxide) with a loss of mass and the formation of so-called “bound air”. This air, as you might guess, was carbon dioxide.
For the first time, with a detailed study of CO2, Joseph Black proved that the air around us is not a single substance, but a mixture of gases. Until this moment, all scientists considered air to be one gas.
Application of carbon
Food additive E290 (carbon dioxide) is approved for use in the food industry, as a preservative, acidity regulator, antioxidant, gasification of drinks (alcoholic and non-alcoholic). Also, dissolved carbon dioxide particles in water have a disinfecting and antibacterial effect.
Food industry
In bakery production, the food additive E290 is a leavening agent that gives products fluffiness and volume. In winemaking, carbonic acid is a fermentation regulator.
Carbon dioxide in crystallized form is used for transporting and storing food products.
The food additive E290 in optimal quantities does not adversely affect the body, therefore it can be used for the manufacture of food products.
Chemical industry
The chemical industry uses CO₂ to regulate the temperature of the reactor, produce synthetic chemicals, neutralize the alkali of wastewater and increase its conductivity, drying\purifying polymers of plant and animal origin.
Medicine, cosmetology
For medicinal purposes, carbon dioxide is used to stimulate deep breathing and cryoablation of malignant tumors.
In dermatocosmetology, carbon dioxide is injected subcutaneously to break down lipids. When an isotonic solution enters the dermis, it reacts with carbon dioxide, breaks down into bicarbonates and hydrogen ions, leading to fat burning. Remaining CO₂ causes a lack of oxygen, stimulating improved blood flow and metabolism.
Metallurgy
Metallurgy uses carbon dioxide to protect the environment from harmful emissions into the atmosphere:
- smoke deposition and nitrogen minimization when opening industrial furnaces, bottom mixing;
- for smoke deposition during transportation of ingots and matte of non-ferrous metals;
- when draining mine acid water for liquid recirculation.
The metallurgical industry uses lasers to cut metals; the devices are charged with liquid carbon dioxide. Thus, carbon dioxide also acts as a fuel for metallurgical equipment.
Laboratory research
Mobile carbon dioxide (stage between liquid and gaseous) is used for extraction and chromatographic analysis.
Printing
Carbon dioxide is used to regulate the pH of processed raw materials, bleach wood, and cellulose for the high-quality production of notebooks and white sheets. And also to improve equipment performance, neutralize tall oil.
Electronics
Carbon dioxide in electronics is used to test the environmental impact of electronic equipment. Also for increasing conductivity, wastewater treatment, eliminating photohardening particles from crystal plates to avoid the use of organic type solvents.
Environmental protection
Carbon dioxide is used as an alternative to sulfuric acid to maintain the pH of wastewater.
Effect on the human body
Carbon dioxide is the end product of metabolism that occurs during glucose-fat breakdown. Hemoglobin carries it to the lungs, from where it is released into the air. Accumulation in the body leads to expansion of capillaries, increased blood flow, and excess oxygen.
Benefits of carbon dioxide
It is carbon dioxide that excites the respiratory system, so the next breath is regulated by the amount of carbon dioxide. When you inhale, an exchange occurs - the intake of oxygen and the release of carbon dioxide.
Note! Oxygen is transported through cells using hemoglobin, and waste (in the form of CO₂ gas) is transported by it to the respiratory system for elimination.
Processes in which CO₂ gas is involved:
- metabolic support;
- excitability of the nervous system;
- regulation of the vascular-motor center, breathing;
- electrolyte composition of blood;
- activity of hormones and enzymes.
Carbon dioxide helps oxygen to be released and enter the body's cells. A lack of CO₂ leads to difficulty breathing, a feeling of lack of oxygen, lethargy, poor health, and malfunction of the entire body.
Harm
The body needs carbon dioxide along with oxygen, but its lack or excess leads to serious problems, such as:
- Hypocapnia. The first manifestations of nausea: dizziness. If left untreated, fainting occurs. It is characterized by a lack of carbon dioxide in the body, due to which it is not able to release oxygen molecules and supply the required amount of useful substances.
- Hypercapnia. The level of carbon dioxide in the body exceeds 1000 ppm, causing poisoning. First symptoms: nausea: loss of performance, difficulty breathing, headache. Lack of treatment leads to lack of oxygen and fainting.
Normally, O₂ and CO₂ gases are attached to opposite sides of hemoglobin and move throughout the body, saturating with necessary substances and taking away waste ones. Excess CO₂ interferes with the ability of red blood cells to attach oxygen, causing nutrients to stop saturating the cells.
Increased concentrations of CO₂ gas are found in mines and in everyday life, especially in the autumn and spring, when rooms are ventilated less frequently.
It is believed that drinks containing carbon dioxide wash out calcium and disrupt metabolism, therefore they are contraindicated for people with diseases of the gastrointestinal tract (ulcers, gastritis, etc.)
Hazard and toxicity class
Carbon dioxide is a non-explosive, non-flammable, non-combustible substance. In gaseous form it is 1.5 times heavier than air, so it accumulates in mines, tunnels, pits, and inside underground equipment.
When the skin comes into contact with carbon dioxide, the affected area may tingle, redness, a feeling of warmth, even frostbite. Slow warming with cold water leads to progression of the disease by an external source of cold.
Hot water can cause burns to the affected area due to partial/complete loss of temperature sensitivity of the dermis. Contact your doctor immediately!
Reservoirs with CO₂ gas can explode when exposed to high temperatures, impacts and sublimation of liquefied acid, or depressurization of the cylinder.
Basic physical properties of carbon dioxide at different temperatures
| Temperature | Density, ρ | Specific heat capacity, Cp | Thermal conductivity, λ | Kinematic viscosity, ν | Prandtl number, Pr |
| K | kg/m3 | J/(kg • K) | W/(m • K) | (m2/s) x 10-6 | — |
| 280 | 1,902 | 830 | 0,0152 | 7,36 | 0,765 |
| 300 | 1,773 | 851 | 0,0166 | 8,40 | 0,766 |
| 400 | 1,326 | 942 | 0,0243 | 14,30 | 0,737 |
| 500 | 1,059 | 1020 | 0,0325 | 21,80 | 0,725 |
| 600 | 0,883 | 1080 | 0,0407 | 30,60 | 0,717 |
| 700 | 0,756 | 1130 | 0,0481 | 40,30 | 0,717 |
* Tabular data was prepared based on materials from foreign reference books
Carbon dioxide storage and transportation
High-pressure cylinders with carbon dioxide are stored in specialized warehouses and fenced areas equipped with a canopy that prevents the tanks from coming into contact with sunlight and precipitation. Store for no more than 1 year from the date of manufacture.
Low-temperature carbon dioxide with liquid substance is stored in isothermal tanks. Shelf life 6 months from date of manufacture.
Carbon dioxide in cylinders
Transportation of carbon dioxide belongs to the 2nd hazard class. Strict adherence to safety rules is required: do not throw, do not hit! To avoid damage to the cylinder, gas leakage and, as a result, explosion!
Carbon dioxide in nature natural sources
These sources include oxidative processes of varying intensity:
- Respiration of living organisms. From the school course in chemistry and botany, everyone remembers that during photosynthesis, plants absorb carbon dioxide and release oxygen. But not everyone remembers that this happens only during the day, with a sufficient level of lighting. In the dark, plants, on the contrary, absorb oxygen and release carbon dioxide. So trying to improve the air quality in a room by turning it into thickets of ficus and geraniums can play a cruel joke.
- Eruptions and other volcanic activity. CO2 is emitted from the depths of the Earth's mantle along with volcanic gases. In the valleys near the sources of eruptions there is so much gas that, accumulating in the lowlands, it causes suffocation of animals and even people. There are several known cases in Africa when entire villages were suffocated.
- Combustion and rotting of organic matter. Combustion and rotting are the same oxidation reaction, but occurring at different rates. Carbon-rich decaying organic matter from plants and animals, forest fires and smoldering peatlands are all sources of carbon dioxide.
- The largest natural reservoir of CO2 is the waters of the world's oceans, in which it is dissolved.
Carbon dioxide in nature
Over millions of years of evolution of carbon-based life on Earth, many billions of tons of carbon dioxide have accumulated in various sources. Its immediate release into the atmosphere will lead to the death of all life on the planet due to the impossibility of breathing. It’s good that the probability of such a one-time release tends to zero.
Where to buy, how much does carbon dioxide cost?
You can purchase carbon dioxide for industrial purposes:
- at the manufacturer's office;
- at the distributor
- through the Internet.
When purchasing from a factory or distributor, you will place an order for the required amount of liquefied carbon dioxide. Delivery is organized by the sender, observing safety precautions. The price is negotiable. It is negotiated directly when placing an order, based on the form of the substance (liquid\"dry ice") and the size of the order.
Carbon dioxide does not have a negative effect on the body if consumed in reasonable quantities. Combines with many elements, helping to create new substances used in everyday life and industry.